��Ŀ����
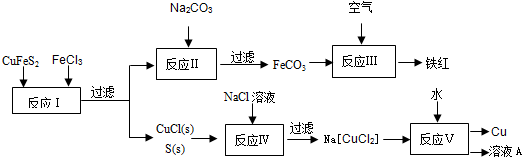
��ͭ��CuFeS2������ȡͭ���仯�������Ҫԭ��֮һ������¯������Ҫ�ɷ� ��Fe0��Fe203��Si02��Al203��������������ת����ϵ����ش�
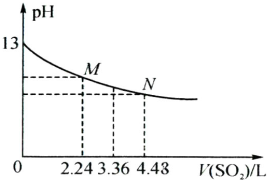
��1��д����֤��SO2�������������������ԵĻ�ѧ����ʽ______o
��2����NaOH��Һ����SO2������NaHSO3��ҺpH��7�������Һ�д������ӵ����ʵ��� Ũ���ɴ�С��˳����______��
��3��д��Cu2S������ȡ��ͭ�Ļ�ѧ����ʽ______
��4���ϵ��Һ�г�����Pb2+��Zn2+����ϵ��Һ�м���Na2S��Һ������PbS��ZnS����ʱ��C��Zn2+����C��Pb2+��=______����֪��Ksp��Pbs��=3.4��10-28mol?L-2
���� Ksp��Zns��=1.6��10-24mol?L-2��
��5��д��֤����ҺI�к���Fe2+��ʵ�����______o
��6��Na2FeO4��ɱ����ˮ��ԭ����______
��7��Na2FeO4��Zn������ɼ��Ե�أ��䷴ӦʽΪ��3Zn+2FeO42-+8H20�T3Zn��OH��2+2Fe��0H����+40H-o��д���ŵ�ʱ�����缫��Ӧʽ______��

�⣺��1�����������������ˮ��Һ������Ӧ���ɵ����������SO2+2H2S=3S��+2H2O��SO2���������������������ԣ��ʴ�Ϊ��SO2+2H2S=3S��+2H2O��
��3��NaHSO3��Һ������������������۵��뻹��ˮ�ⶼ�ǽ����ģ�NaHSO3��Һ�����ԣ�˵���������������ˮ��̶�С�ڵ���̶ȣ�����c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-�����ʴ�Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��2����ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ��Ԫ�ػ��ϼ�ͭԪ�ػ��ϼ۽��ͣ���XYZԪ�ػ��ϼ����ߣ���������Ԫ�ػ��ϼ۽��ͣ�˵����ͭ����ԭ����Ԫ�ر�������������������ԭ��ӦΪCu2S+O2 2Cu+SO2���ʴ�Ϊ��Cu2S+O2
2Cu+SO2���ʴ�Ϊ��Cu2S+O2 2Cu+SO2��
2Cu+SO2��
��4��C��Zn2+����C��Pb2+��=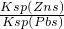 =
= =4.7��10-3��
=4.7��10-3��
��5������KMnO4��Һ������Fe2+����Һ��ɫ��ȥ���ʴ�Ϊ��ȡ������Һ���Թ��У�Ȼ��μ���������KMnO4��Һ����ɫ��ȥ��֤����Fe2+��
��6��Na2FeO4�ǿ�����ˮ��ǿ����������ˮ����ɱ�����������ã��仹ԭ������Ҫ��Fe3+����ˮ���γ�Fe��OH��3���壬Fe��OH��3���壬������ˮ�е������Ĺ���������ʴ�Ϊ��Na2FeO4�ǿ�����ˮ��ǿ����������ˮ����ɱ�����������ã��仹ԭ������Ҫ��Fe3+����ˮ���γ�Fe��OH��3���壬Fe��OH��3���壬������ˮ�е������Ĺ��������
��7����Ӧԭ��Ϊ��3Zn+2FeO42-+8H20�T3Zn��OH��2+2Fe��0H��+40H-�������缫��ӦʽΪ��FeO42-+3e��+4H2O�TFe��OH��3+5OH�����ʴ�Ϊ��FeO42-+3e��+4H2O�TFe��OH��3+5OH����
��������1�����ݶ��������������ˮ��Һ������Ӧ���ɵ����������
��2��NaHSO3��Һ�����ԣ�˵���������������ˮ��̶�С�ڵ���̶ȣ�Ȼ����ݵ���ƽ���ˮ��ƽ��������
��3����ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ��˵����ͭ����ԭ����Ԫ�ر�����������������ԭ��Ӧ�Ļ��ϼ������غ������д�жϣ�
��4������C��Zn2+����C��Pb2+��=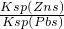 ��
��
��5����������KMnO4��Һ������Fe2+����Һ��ɫ��ȥ��
��6������Na2FeO4�ǿ�����ˮ��ǿ����������ˮ����ɱ�����������ã����仹ԭ������Ҫ��Fe3+����ˮ���γ�Fe��OH��3���壬Fe��OH��3���壬������ˮ�е������Ĺ��������
��7������ԭ��ط�Ӧ��3Zn+2FeO42-+8H2O=3Zn��OH��2+2Fe��OH��3+4OH����������FeO42-+������ԭ��Ӧ��
���������⿼��ѧ���Ķ���Ŀ��ȡ��Ϣ��������������ԭ��Ӧ��ԭ��صĹ���ԭ����Ӧ�ã��缫��Ӧ���缫������жϵȣ��Ѷ��еȣ�Ҫ��ѧ��Ҫ����ʵ�Ļ���֪ʶ���������֪ʶ��������������ע�����֪ʶ��ȫ�����գ�
��3��NaHSO3��Һ������������������۵��뻹��ˮ�ⶼ�ǽ����ģ�NaHSO3��Һ�����ԣ�˵���������������ˮ��̶�С�ڵ���̶ȣ�����c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-�����ʴ�Ϊ��c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-����
��2����ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ��Ԫ�ػ��ϼ�ͭԪ�ػ��ϼ۽��ͣ���XYZԪ�ػ��ϼ����ߣ���������Ԫ�ػ��ϼ۽��ͣ�˵����ͭ����ԭ����Ԫ�ر�������������������ԭ��ӦΪCu2S+O2
 2Cu+SO2���ʴ�Ϊ��Cu2S+O2
2Cu+SO2���ʴ�Ϊ��Cu2S+O2 2Cu+SO2��
2Cu+SO2����4��C��Zn2+����C��Pb2+��=
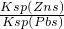 =
= =4.7��10-3��
=4.7��10-3����5������KMnO4��Һ������Fe2+����Һ��ɫ��ȥ���ʴ�Ϊ��ȡ������Һ���Թ��У�Ȼ��μ���������KMnO4��Һ����ɫ��ȥ��֤����Fe2+��
��6��Na2FeO4�ǿ�����ˮ��ǿ����������ˮ����ɱ�����������ã��仹ԭ������Ҫ��Fe3+����ˮ���γ�Fe��OH��3���壬Fe��OH��3���壬������ˮ�е������Ĺ���������ʴ�Ϊ��Na2FeO4�ǿ�����ˮ��ǿ����������ˮ����ɱ�����������ã��仹ԭ������Ҫ��Fe3+����ˮ���γ�Fe��OH��3���壬Fe��OH��3���壬������ˮ�е������Ĺ��������
��7����Ӧԭ��Ϊ��3Zn+2FeO42-+8H20�T3Zn��OH��2+2Fe��0H��+40H-�������缫��ӦʽΪ��FeO42-+3e��+4H2O�TFe��OH��3+5OH�����ʴ�Ϊ��FeO42-+3e��+4H2O�TFe��OH��3+5OH����
��������1�����ݶ��������������ˮ��Һ������Ӧ���ɵ����������
��2��NaHSO3��Һ�����ԣ�˵���������������ˮ��̶�С�ڵ���̶ȣ�Ȼ����ݵ���ƽ���ˮ��ƽ��������
��3����ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ��˵����ͭ����ԭ����Ԫ�ر�����������������ԭ��Ӧ�Ļ��ϼ������غ������д�жϣ�
��4������C��Zn2+����C��Pb2+��=
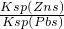 ��
����5����������KMnO4��Һ������Fe2+����Һ��ɫ��ȥ��
��6������Na2FeO4�ǿ�����ˮ��ǿ����������ˮ����ɱ�����������ã����仹ԭ������Ҫ��Fe3+����ˮ���γ�Fe��OH��3���壬Fe��OH��3���壬������ˮ�е������Ĺ��������
��7������ԭ��ط�Ӧ��3Zn+2FeO42-+8H2O=3Zn��OH��2+2Fe��OH��3+4OH����������FeO42-+������ԭ��Ӧ��
���������⿼��ѧ���Ķ���Ŀ��ȡ��Ϣ��������������ԭ��Ӧ��ԭ��صĹ���ԭ����Ӧ�ã��缫��Ӧ���缫������жϵȣ��Ѷ��еȣ�Ҫ��ѧ��Ҫ����ʵ�Ļ���֪ʶ���������֪ʶ��������������ע�����֪ʶ��ȫ�����գ�

��ϰ��ϵ�д�
�����Ŀ
��ͭ��CuFeS2������ȡͭ���仯�������Ҫԭ��֮һ����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭʱ�������·�Ӧ��2Cu2O+Cu2S
6Cu+SO2����
��Ӧ������SO2�Ǵ�����Ⱦ�����NaOH��Һ���յõ�NaHSO3�������£�0.1mol?L-1NaHSO3��Һ��pHС��7������Һ��c��H2SO3�� c��SO32-�������������=����������ԭ���� ��
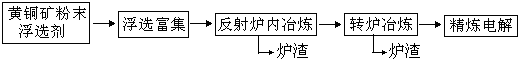
��ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ƣ�
��1����������д�ͭ���õ���ͭ��ԭ���������� ��������
��2������ͭ�����ķ�Һ�к���Fe2+��Fe3+��Cu2+�Ƚ��������ӣ���֪25��ʱ�������ݣ�������������⣺
��25���£���Ũ�Ⱦ�Ϊ0.1mol?L-1��FeCl2��CuCl2�����Һ����μ��백ˮ�������� �������ѧʽ�������ɸó��������ӷ���ʽΪ ��
| ||
��Ӧ������SO2�Ǵ�����Ⱦ�����NaOH��Һ���յõ�NaHSO3�������£�0.1mol?L-1NaHSO3��Һ��pHС��7������Һ��c��H2SO3��
��ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ƣ�
��1����������д�ͭ���õ���ͭ��ԭ����������
��2������ͭ�����ķ�Һ�к���Fe2+��Fe3+��Cu2+�Ƚ��������ӣ���֪25��ʱ�������ݣ�������������⣺
| ���� | Fe��OH��2 | Cu��OH��2 | Fe��OH��3 |
| Ksp | 8.0��10-16 | 2.2��-20 | 4.0��10-38 |