��Ŀ����
��ͭ��CuFeS2������ȡͭ���仯�������Ҫԭ��֮һ����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭʱ�������·�Ӧ��2Cu2O+Cu2S
| ||
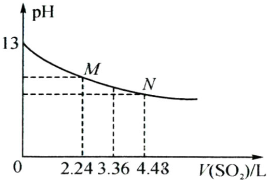
��Ӧ������SO2�Ǵ�����Ⱦ�����NaOH��Һ���յõ�NaHSO3�������£�0.1mol?L-1NaHSO3��Һ��pHС��7������Һ��c��H2SO3��
��ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ƣ�
��1����������д�ͭ���õ���ͭ��ԭ����������
��2������ͭ�����ķ�Һ�к���Fe2+��Fe3+��Cu2+�Ƚ��������ӣ���֪25��ʱ�������ݣ�������������⣺
| ���� | Fe��OH��2 | Cu��OH��2 | Fe��OH��3 |
| Ksp | 8.0��10-16 | 2.2��-20 | 4.0��10-38 |
�����������£�0.1mol?L-1NaHSO3��Һ��pHС��7��˵��HSO3-����̶ȴ���ˮ��̶ȣ�
��1��������ͬʱ����ͭΪ��������ͭΪ������
��2���ܶȻ�ԽС��Խ�����ɳ�����
��1��������ͬʱ����ͭΪ��������ͭΪ������
��2���ܶȻ�ԽС��Խ�����ɳ�����
����⣺����֪NaHSO3�е�HSO3-���ܵ�������ˮ�⣺HSO3-?H++SO32-���������ԣ�HSO3-+H2O?H2SO3 +OH- ˮ���Լ��ԣ������£�0.1mol?L-1NaHSO3��Һ��pHС��7��
˵���������ˮ�⣬�ʣ�c��SO32-����c��H2SO3����
�ʴ�Ϊ������NaHSO3��Һ��HSO3-����̶ȴ���ˮ��̶ȣ�
��1��������ͬʱ����ͭΪ��������ͭΪ�������ʴ�Ϊ����ͭ��
��2��Cu��OH��2���ܶȻ���С����μӰ�ˮʱ��������Cu��OH��2��������Ӧ�����ӷ���ʽΪCu2++2NH3?H2O=Cu��OH��2��+2NH4+��
�ʴ�Ϊ��Cu��OH��2��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��
˵���������ˮ�⣬�ʣ�c��SO32-����c��H2SO3����
�ʴ�Ϊ������NaHSO3��Һ��HSO3-����̶ȴ���ˮ��̶ȣ�
��1��������ͬʱ����ͭΪ��������ͭΪ�������ʴ�Ϊ����ͭ��
��2��Cu��OH��2���ܶȻ���С����μӰ�ˮʱ��������Cu��OH��2��������Ӧ�����ӷ���ʽΪCu2++2NH3?H2O=Cu��OH��2��+2NH4+��
�ʴ�Ϊ��Cu��OH��2��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��
���������⿼���Ϊ�ۺϣ��漰�����������Ⱦ��������ͭ�ľ����Լ����ܵ���ʵ��ܽ�ƽ�����⣬Ϊ�߿��������ͣ�������ѧ���ķ��������Ŀ��飬ע����ػ���֪ʶ��ѧϰ���ѶȲ���

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ