��Ŀ����
6�������루Be�����仯����������ƵĻ�ѧ���ʣ���֪��BeCl2+Na2BeO2+2H2O�T2NaCl+2Be��OH��2������ȫ���У��������ƶ���ȷ���ǣ�������| A�� | Be��OH��2�����������ᣬ��������NaOH��Һ | |
| B�� | BeCl2ˮ��Һ�ĵ�����ǿ����BeCl2�����ӻ����� | |
| C�� | Na2BeO2��Һ��pH��7���������ɲ����պ�õ��IJ�����ΪBeO | |
| D�� | BeCl2��Һ��pH��7���������ɲ����պ�õ��IJ����������BeCl2 |
���� �����ڱ���Be��Alλ�ڶԽ���λ�ã��������ƣ��ɷ�ӦBeCl2+Na2BeO2+2H2O=2NaCl+2 Be��OH��2����֪��BeCl2��Na2BeO2��������ˮ������Be��OH��2���������Ȼ�����ƫ�����Ƶķ�Ӧ�������������������������������ж�Be�������롢Be��OH��2���ʣ�
��� �⣺�����ڱ���Be��Alλ�ڶԽ���λ�ã��������ƣ��ɷ�ӦBeCl2+Na2BeO2+2H2O=2NaCl+2 Be��OH��2����֪��BeCl2��Na2BeO2��������ˮ������Be��OH��2���������Ȼ�����ƫ�����Ƶķ�Ӧ�������������������������������ж�Be�������롢Be��OH��2���ʣ�
A��Be��OH��2���������������������������ԣ�������������ᣬ��������NaOH��Һ����A��ȷ��
B�����ݻ�����������״̬���ܷ����ж����Ƿ������ӻ�������ۻ�������ˮ��Һ��Ҳ���Ե��磬BeCl2ˮ��Һ������ǿ������˵��BeCl2�����ӻ������B����
C��Na2BeO2��Һˮ��ʼ��ԣ���Һ��pH��7���������ɣ�ˮ��õ���������������Be��OH��2��Ӧ�õ�Na2BeO2�����պ�ɵò�����Na2BeO2����C����
D��BeCl2Ϊǿ�������Σ�ˮ������ԣ���Һ��pH��7���������ɣ�HCl�ӷ���ˮ�⳹�ף��õ�Be��OH��2�����պ�ɵò�����BeO����D����
��ѡA��
���� ���⿼��Ԫ�������ɣ�Ϊ��Ƶ���㣬��ȷ�������ʼ�Ԫ���������ǽⱾ��ؼ�������֪ʶǨ�Ƶķ��������������Ŀ�ѶȲ���

| A�� | ��$\stackrel{-1}{Cl}$ת��$\stackrel{+5}{Cl}$����6e- | B�� | ��$\stackrel{-1}{Cl}$ת��$\stackrel{+5}{Cl}$����5e- | ||
| C�� | ��$\stackrel{0}{Cl}$ת��$\stackrel{+5}{Cl}$����6e- | D�� | ��$\stackrel{0}{Cl}$ת��$\stackrel{+5}{Cl}$����5e- |
| A�� |  ������ͼװ�ý�����Ȫʵ�� | B�� | 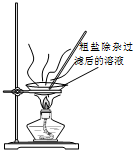 ������ͼװ�õõ����� | ||
| C�� | 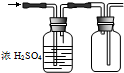 ������ͼװ�ø��ﲢ�ռ�SO2 | D�� | 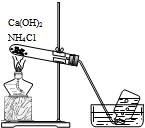 ������ͼװ����ȡ���ռ�NH3 |
| A�� | Ħ����һ������������6.02��1023������ | |
| B�� | ���ʵ����ǹ��ʵ�λ�Ƶ��߸�������λ֮һ | |
| C�� | Ħ���������ʵ����ĵ�λ���������ӵ�������λ | |
| D�� | Ħ���Ļ���0.012 Kg 12C��������̼ԭ���� |
����ͨ©�� ����Ͳ ��������ƿ �ܵζ��� �ݷ�Һ©�� ������ƿ��
| A�� | �ۢݢ� | B�� | �ڢݢ� | C�� | �٢ۢ� | D�� | �ڢܢ� |
| A�� | ��������ҺӦ�����ڴ���Ƥ�����Լ�ƿ�� | |
| B�� | NaCl��Һ�����ᾧʱ�����������о���������ʣ������Һ�弴ֹͣ���� | |
| C�� | Һ���ӷ����ڴ��Һ����Լ�ƿ��Ӧ��ˮҺ�� | |
| D�� | ��ˮ�еμ���������FeCl3��Һ���γɴ���Ľ��壬����������ǿ |
| A�� | BaCl2��Ba��NO3��2��NaCl | B�� | Ba��NO3��2��KCl��MgCl2 | ||
| C�� | Na2CO3��BaCl2��MgCl2 | D�� | Na2CO3��K2CO3��NaCl |
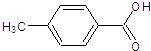 �ж���ͬ���칹�壬���з����������ಢ���б�����ͬ���칹���У��������֣��������������壩
�ж���ͬ���칹�壬���з����������ಢ���б�����ͬ���칹���У��������֣��������������壩