��Ŀ����
2���й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��أ��״�ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��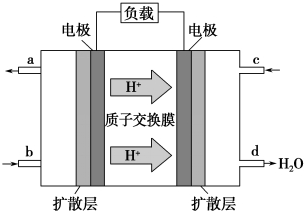
��1���õ�ع���ʱ��c��ͨ�������ΪO2���õ�ظ����ĵ缫��ӦʽΪCH3OH+H2O-6e-�TCO2+6H+��
��2������һ��ʱ���6.4g�״���ȫ��Ӧ����CO2ʱ����1.2NA������ת�ƣ�
��3�����õ��ͨ��O2����Ϊ1L����״�������ҷ�Ӧ��ȫ�����ô˵�������NaCl��Һ�����Ե缫��������ܲ���Cl2�����Ϊ2L����״������
��4���״�ȼ�Ͽ�����CO2������ϳɼ״����÷����ܺõĽ��CO2�������������⣬�������õ�Ӧ��ǰ������֪4.4g CO2������H2���徭����������CH3OH�����ˮ����ʱ�ų�4.95kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ��CO2��g��+3H2��g���TCH3OH��g��+H2O��g����H=-49.5 kJ/mol��
��5����4���еķ�Ӧ����270�桢8MPa���ʵ������������£�CO2��ת���ʴﵽ22%����4.48m3�����ۺ�Ϊ��״������CO2�ܺϳ�CH3OH��������ʵ�����44mol���˹������ܷų�����2178 kJ��
���� ��1�������������ƶ�����֪���Ҳ�缫Ϊ������ͨ�����������õ��ӵĻ�ԭ��Ӧ�����缫Ϊ������������ͨ��ȼ�ϣ�����ʧ���ӵ�������Ӧ��
��2�����ݼ״��缫�����ķ�Ӧ��ת�Ƶ���֮��Ĺ�ϵʽ���㣻
��3������ת�Ƶ����غ�������������������
��4������������������㻯ѧ����ʽ�е�����Ӧ�µķ�Ӧ���ȣ�����Ȼ�ѧ����ʽ����д��������ע���ʾۼ�״̬����Ӧ���ķ�Ӧ�ȣ�
��5������ת���ʽ���Ȼ�ѧ����ʽ����õ���
��� �⣺��1���پ��������ƶ�����֪���Ҳ�缫Ϊ����������ͨ����������c��ͨ�������Ϊ���������缫Ϊ������������ͨ��ȼ�ϼ״���������ӦΪ��CH3OH+H2O-6e-�TCO2+6H+��
�ʴ�Ϊ��O2��CH3OH+H2O-6e-�TCO2+6H+��
�������������õ��Ӻ������ӷ�Ӧ����ˮ���缫��ӦʽΪ��3O2+12H++12e-=6H2O�������ϼ״�ʧ���Ӻ�ˮ��Ӧ���ɶ�����̼�������ӣ��缫��ӦʽΪ2CH3OH-12e-+2H2O=2CO2��+12H+������2CH3OH-12e-+2H2O=2CO2+12H+֪���״���ת�Ƶ���֮��Ĺ�ϵʽ�ã���6.4g�״���ȫ��Ӧ����CO2ʱ��ת�Ƶ��ӵ����ʵ���=$\frac{6.4g}{32g/mol}$��6=1.2mol����ת�Ƶ��Ӹ���Ϊ1.2NA��
�ʴ�Ϊ��1.2��
��3���õ��ͨ��O2�ĵ缫������Ӧ��O2+4H++4e-=2H2O�����������ĵ缫�Ϸ�����Ӧ��2Cl--2e-=Cl2��������ת�Ƶ����غ�����������=$\frac{1L��4}{2}$=2L���ʴ�Ϊ��2��
��4����֪4.4g CO2������H2������������CH3OH�����ˮ����ʱ�ų�4.95kJ����������1mol������̼ȫ����Ӧ����49.5KJ������Ȼ�ѧ����ʽ��д����д���Ȼ�ѧ����ʽΪ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.5KJ/mol��
�ʴ�Ϊ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.5KJ/mol��
��5����270�桢8MPa���ʵ������������£�����Ϊ���壬��Ӧ���Ȼ�ѧ����ʽΪ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.5KJ/mol��CO2��ת���ʴﵽ22%����4.48m3�����ۺ�Ϊ��״������CO2�ںϳ�CH3OH���������ת�������ʵ���Ϊ��$\frac{4.48��1{0}^{3}L}{22.4L/mol}$��22%=44mol����Ӧ�ܷų�����44mol��49.5KJ/mol=2178KJ��
�ʴ�Ϊ��200��2178��
���� ���⿼����ԭ��صĹ���ԭ���Լ��缫��Ӧʽ����д���㡢�Ȼ�ѧ����ʽ����д����㡢�Ȼ�ѧ����ʽ�ĺ��塢����Ӧ�ã���˹���ɵļ���Ӧ�ã���Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��������ˮ��v��H2������ | B�� | ����CH3COONa���壬v��H2����С | ||
| C�� | ����NH4HSO4���壬v��H2������ | D�� | �μ�����CuSO4��Һ��v��H2����С |
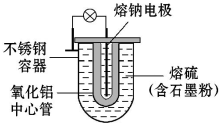 ij������˾�״η�����Na-S��ص��йر�������ṹ��ͼ��ʾ����ط�ӦΪ2Na+$\frac{n}{8}$S8$?_{���}^{�ŵ�}$Na2Sn������˵������ȷ���ǣ�������
ij������˾�״η�����Na-S��ص��йر�������ṹ��ͼ��ʾ����ط�ӦΪ2Na+$\frac{n}{8}$S8$?_{���}^{�ŵ�}$Na2Sn������˵������ȷ���ǣ�������| A�� | ���ʱ���Ƶ缫���Դ�ĸ������� | |
| B�� | �ŵ�ʱNa+�������ƶ� | |
| C�� | �ŵ�ʱ���Ƶ缫����ص����� | |
| D�� | ���ʱ������ӦʽΪ8Sn2--16e-�TnS8 |
| A�� | ʹ��̪��Һ������Һ��Na+��Cl-��SO42-��Fe3+ | |
| B�� | ʹ��ɫʯ����Һ������Һ��Fe2+��Mg2+��CO32-��Cl- | |
| C�� | ̼��������Һ��K+��SO42-��Cl-��H+ | |
| D�� | pH��7����Һ��K+��Ba2+��Cl-��NO3- |
| A�� | ��״���º���NA����ԭ�ӵĺ��������ԼΪ11.2 L | |
| B�� | ��0.01 mol FeCl3�ı�����Һ������ڵ�ˮ�У��Ƶõ�������������������Ϊ0.01NA | |
| C�� | ��״���£�22.4 L CCl4�����ķ�����ԼΪNA | |
| D�� | CO��N2��ɵ�42g���������ԭ�ӵĸ���Ϊ3NA |
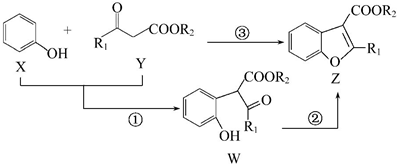
�������й���������ȷ���ǣ�������
| A�� | ��Ӧ�����ڼӳɷ�Ӧ | B�� | 1 mol W��ȫ�ӳ���Ҫ4 mol H2 | ||
| C�� | X�ĺ˴Ź����������ĸ��� | D�� | X��Y��W��Z������NaOH��Һ��Ӧ |