��Ŀ����
����Ŀ��������Ԫ��X��Y��Z��W��ԭ�����������������У�XԪ��ԭ�ӵ��������������ڲ��������2����X��Z�ĺ˵����֮��Ϊ3��4��W��Z�����WZ2��WZ3���ֻ���������ֻ�����֮���ͨ�����淴Ӧ�ת�����ش��������⣺
��1��ZԪ����Ԫ�����ڱ��е�λ����________________��WԪ��ԭ�ӵĽṹʾ��ͼΪ____________________��
��2��ʵ������ȡYԪ����̬�⻯��Ļ�ѧ����ʽΪ________________________��
��3�����������£�WԪ������������Ӧˮ�����Ũ��Һ��XԪ�صĵ��ʷ�����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ________________________���ռ���Ӧ���������壬ijͬѧ�����ͼװ�ü����������ɣ��Լ�������������Aƿ�е��Լ���_____________________��

���𰸡� ��2���ڵ�VIA�� 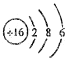 2NH4Cl + Ca(OH)2
2NH4Cl + Ca(OH)2![]() CaCl2+ 2NH3��+2H2O C+2H2SO4(Ũ)
CaCl2+ 2NH3��+2H2O C+2H2SO4(Ũ) ![]() CO2��+2SO2��+2H2O Ʒ����Һ
CO2��+2SO2��+2H2O Ʒ����Һ
��������������Ԫ��X��Y��Z��W��ԭ�����������������У�XԪ��ԭ�ӵ��������������ڲ��������2�������ڲ������Ϊ2������������Ϊ4��ΪCԪ����X��Z�ĺ˵����֮��Ϊ3��4����ZΪOԪ�أ����YΪNԪ����W��Z�����WZ2��WZ3���ֻ���������ֻ�����֮���ͨ�����淴Ӧ�ת������WΪSԪ�ء�
(1)OԪ����Ԫ�����ڱ���λ�ڵ�2���ڣ��ڢ�A����SԪ��ԭ�ӵĽṹʾ��ͼΪ![]() ���ʴ�Ϊ����2���ڣ��ڢ�A����
���ʴ�Ϊ����2���ڣ��ڢ�A����![]() ��
��
(2)ʵ������ȡ�����Ļ�ѧ����ʽΪ2NH4Cl + Ca(OH)2![]() CaCl2 + 2NH3��+2H2O���ʴ�Ϊ��2NH4Cl + Ca(OH)2
CaCl2 + 2NH3��+2H2O���ʴ�Ϊ��2NH4Cl + Ca(OH)2![]() CaCl2 + 2NH3��+2H2O��
CaCl2 + 2NH3��+2H2O��
(3)���������£�Ũ������̼�ܹ�������Ӧ����Ӧ�Ļ�ѧ����ʽΪ2H2SO4(Ũ) + C![]() CO2��+ 2H2O + 2SO2�������������̼�Ͷ���������Ҫ���Ⱦ��ж�������Ȼ���������������ټ��������̼�����Aƿ��Ӧ��ʢ��Ʒ����Һ��Bƿ�л���Ʒ����Һ������������Ƿ������Cƿ���ó���ʯ��ˮ���������̼���ʴ�Ϊ��2H2SO4(Ũ) + C
CO2��+ 2H2O + 2SO2�������������̼�Ͷ���������Ҫ���Ⱦ��ж�������Ȼ���������������ټ��������̼�����Aƿ��Ӧ��ʢ��Ʒ����Һ��Bƿ�л���Ʒ����Һ������������Ƿ������Cƿ���ó���ʯ��ˮ���������̼���ʴ�Ϊ��2H2SO4(Ũ) + C![]() CO2��+ 2H2O + 2SO2����Ʒ����Һ��
CO2��+ 2H2O + 2SO2����Ʒ����Һ��

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�����Ŀ��I .������Ԫ��X��Y��Z��W�����ڱ��е����λ����ͼ��ʾ������WԪ�ص�ԭ�ӽṹʾ��ͼΪ![]() ��
��
W | X | Y |
Z |
��ش��������⣺
��1��ZԪ����Ԫ�����ڱ��е�λ����________________________________________��
��2��X��Y��Z����Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_________________��Ԫ�ط��ű�ʾ����
��3��X��Z��W����Ԫ�ص�����������Ӧˮ�����������ǿ������˳��Ϊ____________���û�ѧʽ��ʾ��
��4���õ���ʽ��ʾWY2���γɹ��̣�__________________________________��
��5��д��W������Ũ���ᷴӦ�Ļ�ѧ����ʽ��__________________________________��
��.A��B��C�������ʴ�����ͼת����ϵ��

��1����BΪ��ɫ��״�������A��C��Ӧ�����ӷ���ʽΪ____________________________��
��2����B��Һ�еμ����Ȼ�����Һ�����������ɫ��������A��C��Ӧ�����ӷ���ʽΪ__________________________________��
����Ŀ����X��Y��Z��T���ֶ�����Ԫ�ء�X��Y��Z��Ԫ�������ڱ��е�λ����ͼ��ʾ����Ԫ�ص�ԭ������֮����41��X��T�ĵ����ڲ�ͬ�����·�Ӧ����������T2X(��ɫ����)��T2X2(����ɫ����)���������ֻ����
X | |
Y | Z |
(1)��Ԫ�صķ�����X______��Y________��Z________��T___________��
(2)Yԭ�ӵĽṹʾ��ͼΪ____________________________________��
(3)�õ���ʽ��ʾY��T��ɵĻ�������γɹ��̣�_________________________��
(4)X��Y���⻯����ȶ���_________�����û�ѧʽ��ʾ����
����Ŀ���������������������ͷ�����ȷ����
ѡ�� | A | B | C | D |
ԭ�Ӿ��� | ʯī | ��ʯ�� | ̼���� | ���ʯ |
���Ӿ��� | �� | ��̬�� | �Ȼ�� | �ɱ� |
���Ӿ��� | ������ | ʳ�� | ���� | ������ |
�������� | ͭ | �� | �� | �� |
A. A B. B C. C D. D

