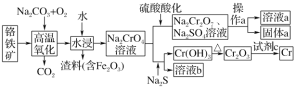
ћвƒњƒЏ»Ё
°Њћвƒњ°њ“‘µнЈџќ™÷ч“™‘≠ЅѕЇѕ≥…“ї÷÷Њя”–єыѕгќґµƒќп÷ CЇЌїѓЇѕќпDµƒЇѕ≥…¬Јѕя»зѕ¬ЌЉЋщ Њ°£
«лїЎірѕ¬Ѕ–ќ ћв£Ї
(1)AµƒљбєєЉт љќ™________£ђBЈ÷„”÷–µƒєўƒ№Ќ≈√ы≥∆ќ™________°£
(2)Јі”¶Ґџµƒїѓ—ІЈљ≥ћ љќ™_______°£
(3)Јі”¶ҐЁµƒїѓ—ІЈљ≥ћ љќ™_________°£
(4)“—÷™Dµƒѕаґ‘Ј÷„”Ѕњќ™118£ђ”–Ћб–‘«“÷їЇђ”–“ї÷÷єўƒ№Ќ≈£ђ∆д÷–ћЉ°Ґ«вЅљ‘™ЋЎµƒ÷ ЅњЈ÷ эЈ÷±рќ™40.68%°Ґ5.08%£ђ∆д”аќ™—х‘™ЋЎ£ђ‘тDµƒїѓ—І љќ™______£ђ∆дљбєєЉт љќ™_______°£
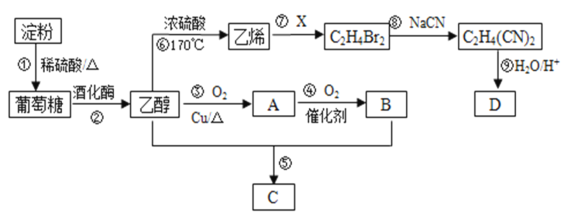
°Њір∞Є°њCH3CHO ф»їщ 2CH3CH2OH£ЂO2![]() 2CH3CHO£Ђ2H2O CH3COOH£ЂCH3CH2OH
2CH3CHO£Ђ2H2O CH3COOH£ЂCH3CH2OH![]() CH3COOCH2CH3£ЂH2O C4H6O4 HOOCCH2CH2COOH
CH3COOCH2CH3£ЂH2O C4H6O4 HOOCCH2CH2COOH
°Њљвќц°њ
µнЈџЊ≠єэЈі”¶Ґў£ђ‘Џѕ°ЅтЋбµƒ„ч”√ѕ¬£ђЋЃљвќ™∆ѕћ—ћ«£ї∆ѕћ—ћ«Њ≠єэЈі”¶ҐЏ£ђ‘ЏЊ∆їѓ√Єµƒ„ч”√ѕ¬…ъ≥…““іЉ£ї““іЉЊ≠єэЈі”¶Ґџ£ђ‘ЏЌ≠µƒіяїѓ„ч”√ѕ¬”л—х∆шЈі”¶…ъ≥…A£ђAќ™““»©£ї““»©Њ≠єэЈі”¶Ґ№£ђЊ≠іяїѓ„ч”√”л—х∆шЈі”¶£ђ…ъ≥…B£ђ‘тBќ™““Ћб£ђ““Ћб”л““іЉЊ≠єэЈі”¶ҐЁ£ђµ√µљC£ђ‘тCќ™““Ћб““х•£ї““іЉЊ≠єэЈі”¶Ґё£ђ‘Џ≈®ЅтЋбµƒ„ч”√ѕ¬£ђ…ъ≥…““ѕ©£ї““ѕ©”лXЊ≠єэЈі”¶Ґя£ђ…ъ≥…C2H4Br2£ђњ…÷™Xќ™Br2£ђЊЁіЋ„чір°£
(1)““іЉƒ№‘ЏЌ≠„чіяїѓЉЅµƒћхЉюѕ¬”л—х∆шЈҐ…ъіяїѓ—хїѓЈі”¶…ъ≥…““»©£ђ‘тAќ™““»©£ђљбєєЉт љќ™CH3CHO£ї““»©ƒ№”л—х∆ш‘ЏіяїѓЉЅµƒћхЉюѕ¬ЈҐ…ъЈі”¶…ъ≥…““Ћб£ђ‘тBќ™““Ћб£ђ““ЋбЈ÷„”÷–µƒєўƒ№Ќ≈√ы≥∆ќ™ф»їщ£ђє ір∞Єќ™£ЇCH3CHO£їф»їщ£ї
(2)Јі”¶Ґџ «““іЉ‘ЏЌ≠µƒіяїѓ„ч”√ѕ¬”л—х∆шЈі”¶…ъ≥…““»©£ђЈі”¶µƒїѓ—ІЈљ≥ћ љќ™2CH3CH2OH£ЂO2![]() 2CH3CHO£Ђ2H2O£ђє ір∞Єќ™£Ї2CH3CH2OH£ЂO2
2CH3CHO£Ђ2H2O£ђє ір∞Єќ™£Ї2CH3CH2OH£ЂO2![]() 2CH3CHO£Ђ2H2O£ї
2CH3CHO£Ђ2H2O£ї
(3)Јі”¶ҐЁ «““Ћб”л““іЉЈҐ…ъх•їѓЈі”¶…ъ≥…““Ћб““х•£ђЈі”¶µƒїѓ—ІЈљ≥ћ љќ™£ЇCH3COOH£ЂCH3CH2OH![]() CH3COOCH2CH3£ЂH2O£ђє ір∞Єќ™£ЇCH3COOH£ЂCH3CH2OH
CH3COOCH2CH3£ЂH2O£ђє ір∞Єќ™£ЇCH3COOH£ЂCH3CH2OH![]() CH3COOCH2CH3£ЂH2O£ї
CH3COOCH2CH3£ЂH2O£ї
(4)“—÷™Dµƒѕаґ‘Ј÷„”Ѕњќ™118£ђћЉ°Ґ«вЅљ‘™ЋЎµƒ÷ ЅњЈ÷ эЈ÷±рќ™40.68%°Ґ5.08%£ђ∆д”аќ™—х‘™ЋЎ£ђ‘тЈ÷„”÷–Їђ”–µƒћЉ‘≠„” эќ™![]() =4£ђЇђ«в‘≠„” эќ™
=4£ђЇђ«в‘≠„” эќ™![]() =6£ђЇђ—х‘≠„” эќ™
=6£ђЇђ—х‘≠„” эќ™![]() =4£ђЋщ“‘DµƒЈ÷„” љќ™C4H6O4£ђЄ√Ј÷„””–Ћб–‘«“÷їЇђ”–“ї÷÷єўƒ№Ќ≈£ђє єўƒ№Ќ≈ќ™ф»їщ£ђ«“Їђ”–ЅљЄц£ђљбєєЉт љќ™£ЇHOOCCH2CH2COOH£ђє ір∞Єќ™£ЇC4H6O4£їHOOCCH2CH2COOH°£
=4£ђЋщ“‘DµƒЈ÷„” љќ™C4H6O4£ђЄ√Ј÷„””–Ћб–‘«“÷їЇђ”–“ї÷÷єўƒ№Ќ≈£ђє єўƒ№Ќ≈ќ™ф»їщ£ђ«“Їђ”–ЅљЄц£ђљбєєЉт љќ™£ЇHOOCCH2CH2COOH£ђє ір∞Єќ™£ЇC4H6O4£їHOOCCH2CH2COOH°£

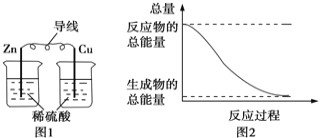
°Њћвƒњ°њ“—÷™Јі”¶2X(g)+Y(g)![]() Z(g)£ђƒ≥—–Њњ–°„йљЂ4moXЇЌ2molY÷√”Џ“ї»Ёїэ≤ї±дµƒ√№±’»Ё∆ч÷–£ђ≤вґ®1minƒЏXµƒ„™їѓ¬ £ђµ√µљµƒ эЊЁ»з±нЋщ Њ£ђѕ¬Ѕ–≈–ґѕ’э»Јµƒ «£® £©
Z(g)£ђƒ≥—–Њњ–°„йљЂ4moXЇЌ2molY÷√”Џ“ї»Ёїэ≤ї±дµƒ√№±’»Ё∆ч÷–£ђ≤вґ®1minƒЏXµƒ„™їѓ¬ £ђµ√µљµƒ эЊЁ»з±нЋщ Њ£ђѕ¬Ѕ–≈–ґѕ’э»Јµƒ «£® £©
t/min | 2 | 4.5 | 5 | 6 |
Xµƒ„™їѓ¬ | 30% | 40% | 70% | 70% |
A.Ћж„≈Јі”¶µƒљш––£ђїмЇѕ∆шћеµƒ√№ґ»≤їґѕ‘ціу
B.Јі”¶‘Џ5.5min ±£ђv’э(X)=vƒж(Z)
C.6min ±£ђ»Ё∆ч÷– £”а1.4 mol Y
D.»фЈі”¶єэ≥ћ÷–£ђ»Ё∆чƒЏ—є«њ≤ї‘ўЄƒ±д£ђњ…“‘Ћµ√чЄ√Јі”¶“—іпµљ∆љЇв„іћђ
°Њћвƒњ°њ≥£ќ¬ѕ¬Љ„»© «“ї÷÷ќё…Ђ°Ґ”–ћЎ віћЉ§–‘∆шќґµƒ∆шће£ђ“„»№”ЏЋЃ£ђ « јљзќј…ъ„й÷ѓ(WHO)»Јґ®µƒ÷¬∞©ќпЇЌ÷¬їы–ќќп÷Ѓ“ї°£ќ“єъєжґ®£ЇЊ” “њ’∆ш÷–Љ„»©µƒ„оЄя»Ё–н≈®ґ»ќ™0.08mg/m3°£ƒ≥—–Њњ–‘—Іѕ∞–°„й…иЉ∆”√»зѕ¬ЈљЈ®≤вґ®ƒ≥Њ” “њ’∆ш÷–Љ„»©µƒЇђЅњ£®Љў…ињ’∆ш÷–ќё∆дЋыїє‘≠–‘∆шће£©£Ї
£®1£©≤вґ®‘≠јн£ЇKMnO4(H+)»№“Їќ™«њ—хїѓЉЅ£ђњ…—хїѓЉ„»©ЇЌ≤ЁЋб£ђЈі”¶µƒјл„”Јљ≥ћ љќ™£Ї
4MnO4-+ 5HCHO + 12H+ == 4Mn2+ + 5CO2°ь+ 11H2O
2MnO4-+ 5H2C2O4 + 6H+ == 2Mn2++10CO2°ь+ 8H2O
£®2£©≤вґ®„∞÷√£Ї≤њЈ÷„∞÷√»зЌЉЋщ Њ£®a°Ґbќ™÷єЋЃЉ–£©
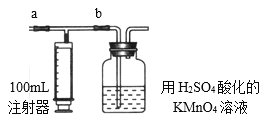
£®3£© µ—й≤љ÷и£Ї
Ґў Љм≤й„∞÷√∆ш√№–‘£®∆ш√№–‘ЅЉЇ√£©°£
ҐЏ ”√___________£®ћо“«∆ч√ы≥∆£©„Љ»Ј“∆»°20.00 mL 2.00°Ѕ10£≠3mol°§L°™1µƒЄя√ћЋбЉЎ»№“Ї£®єэЅњ£©”ЏєгњЏ∆њ÷–≤Ґµќ»л3µќ6mol°§L-1µƒH2SO4»№“Ї±Є”√°£
Ґџ љЂ2.00°Ѕ10-3mol°§L-1µƒ≤ЁЋб±к„Љ»№“Ї÷√”Џ_____________£®ћо“«∆ч√ы≥∆£©÷–±Є”√°£
Ґ№ ітњ™a£ђєЎ±’b£ђ”√„Ґ…д∆ч≥й»°100mL–¬„∞–ёµƒ “ƒЏњ’∆ш°£єЎ±’a£ђітњ™b£ђ‘ўїЇїЇЌ∆ґѓ„Ґ…д∆ч£ђљЂ∆шће»Ђ≤њЌ∆»лЋб–‘Єя√ћЋбЉЎ»№“Ї÷–£ђ є∆д≥дЈ÷Јі”¶°£‘ў»зіЋ÷ЎЄі4іќ£®є≤5іќ£©°£»зєы—єЋЌ∆шће ±Ћўґ»єэњм£ђњ…ƒ№їб≤ъ…ъ ≤√і≤їјыЇуєы£њ___________°£
ҐЁ љЂєгњЏ∆њ÷–µƒ»№“Ї„™»л„ґ–ќ∆њ÷–£®»уѕієгњЏ∆њ2°Ђ3іќ£ђ≤ҐљЂѕіµ”“Ї»Ђ≤њ„™»л„ґ–ќ∆њ£©°£»зєы√ї”–»уѕі£ђЋщ≤вЉ„»©µƒЇђЅњљЂ_________£®ћо°∞∆ЂЄя°±°Ґ°∞∆ЂµЌ°±°Ґ°∞ќё”∞ѕм°±£©£ї
Ґё ”√±к„Љ≤ЁЋб»№“Їµќґ®„ґ–ќ∆њ÷–µƒ»№“Ї£їЉ«¬Љµќґ®ЋщѕыЇƒµƒ≤ЁЋб»№“Їћеїэ°£±Њ µ—й «Јс–и“™÷Є ЊЉЅ£њ_________£®ћо°∞ «їтЈс°±£©£ђµќґ®÷’µг ±µƒ µ—йѕ÷ѕуќ™____________________°£
Ґя ‘ў÷ЎЄі µ—й2іќ°£
£®4£© эЊЁі¶јн£ЇЈ÷±р”Џ„∞–ёЌкє§ЇуµƒµЏ1ћм°ҐµЏ7ћм°ҐµЏ30ћм( “ƒЏ Љ÷’±£≥÷Ќ®Јзїї∆ш„іњц)ґ‘ “ƒЏњ’∆шљш––»°—щ£ђЌ®єэ µ—й≤вµ√“‘ѕ¬»э„й эЊЁ£®√њіќ µ—йЋщ»°µƒKMnO4»№“ЇЊщќ™ 20.00mL£©£Ї
„∞–ёЇу µЏnћм | ≤ЁЋб»№“Ї£®mL£© | Љ„»©µƒ≈®ґ» mg/m3 | |||
1 | 2 | 3 | ∆љЊщ÷µ | ||
1 | 15.86 | 15.72 | 15.67 | 15.75 | |
7 | 26.17 | 26.36 | 26.38 | 26.27 | |
30 | 49.05 | 48.98 | 49.02 | 48.95 | |
…и KMnO4»№“Їµƒќп÷ µƒЅњ≈®ґ»ќ™c1£ђKMnO4»№“Їµƒћеїэќ™v1(mL)£ђ≤ЁЋб»№“Їµƒќп÷ µƒЅњ≈®ґ»ќ™c2£ђ≤ЁЋб»№“Їµƒ∆љЊщћеїэќ™v2(mL)‘т£ЇЉ∆Ћг “ƒЏњ’∆ш÷–Љ„»©≈®ґ»£®mg/m3£©µƒіъ э љќ™__________£®”√c1°Ґc2 °Ґv1°Ґv2±н Њ£©£ђјы”√±н÷– эЊЁЉ∆ЋгµЏ30ћм “ƒЏњ’∆ш÷–Љ„»©≈®ґ»ќ™_______mg/m3°£