��Ŀ����
��ǰ����������һ��ȫ�����ӵ����⣬������������峣��������������CO2��
��Ⱦ������NOx��SOx�ȡ��������Щ����������þͿ��Գ�Ϊ��Ҫ����Դ���Ƚ���˶Ի�������Ⱦ���ֽ���˲�����ԴΣ�����⡣
(1)������̼�ǵ�������ЧӦ��������ף�Ŀǰ���Ǵ���������̼�ķ���֮һ��ʹ����������Ӧ�ϳɼ״����״�������ȼ�ϵ�ص���Ҫȼ�ϡ�CO2��H2��Ӧ�Ʊ�CH3OH��H2O�Ļ�ѧ����ʽΪ
(2)�ڸ�����һ����̼�ɽ���������ԭΪ������
��֪��
��C(s)��O2(g)=CO2(g)��H1����393.5 kJ��mol��1
��CO2(g)��C(s)=2CO(g)��H2����172.5 kJ��mol��1
��S(s)��O2(g)=SO2(g)��H3����296.0 kJ��mol��1
��д��CO��SO2��Ӧ���Ȼ�ѧ����ʽ ��
(3)���᳧���ô���ԭ��������β����CH4�ڴ������¿��Խ�NO2��ԭΪN2��
��֪��CH4(g)��2O2(g)=CO2(g)��2H2O(g)��H����889.6 kJ��mol��1��
N2(g)��2O2(g)=2NO2(g)��H����67.7 kJ��mol��1��
��CH4��ԭNO2����ˮ�����͵������Ȼ�ѧ����ʽ�� ��
(1)CO2��3H2=CH3OH��H2O
(2)2CO(g)��SO2(g)=S(s)��2CO2(g) ��H����270 kJ��mol��1
(3)CH4(g)��2NO2(g)=N2(g)��2H2O(g)��CO2(g) ��H����957.3 kJ��mol��1
�������������ʱӦע���������㣺
(1)��������ҳ���Ӧ��Ͳ��
(2)����Ҫ���ӵ��Ȼ�ѧ����ʽ���д�����
(3)��H����ֵҲҪ���Ȼ�ѧ����ʽ�ĸı���ı䡣

����A��E�������±��е�������ɵģ������½������ʵ���
Һ��1 mLϡ�͵�1 000 mL��pH�ı仯��ϵ��ͼ����ʾ������A��D��Ӧ�õ�E����ش��������⡣
������ | NH4+��H����Na�� |
������ | OH����CH3COO����Cl�� |
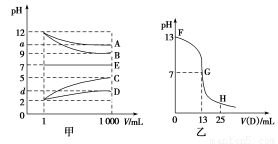
(1)����pH�ı仯��ϵ��д�����ʵĻ�ѧʽ��B ��C ��
(2)д��A��C��Ӧ�����ӷ���ʽ�� ��
(3)ͼ��Ϊ����ʱ��25 mLijŨ�ȵ�B��Һ����εμ�0.2 mol��L��1��D��Һ�Ĺ�����pH�ı仯���ߡ�
��ͼ����B�����ʵ���Ũ��Ϊ mol��L��1��
��G����Һ�����ԣ������ǡ����ȫ��Ӧ�ĵ�����FG���仹��GH���䣿 ���䡣
��FG������Һ�и�����Ũ�ȴ�С��ϵ�� ��
(4)t ��ʱ��A��ϡ��Һ��c(H��)��10��a mol��L��1��c(OH��)��10��b mol��L��1����֪a��b��13�����¶���(t ��)����100 mL 0.2 mol��L��1��C��Һ��100 mL 0.4 mol��L��1��B��Һ��Ϻ�(��Һ����仯���Բ���)����Һ��pH�� ��
���ô�����ʹNO��CO������Ӧ��2NO(g)��2CO(g)??2CO2(g)��N2(g)����H��0��
��֪��������ıȱ���������ѧ��Ӧ���ʡ�Ϊ�˷ֱ���֤�¶ȡ������ıȱ�����Ի�ѧ��Ӧ���ʵ�Ӱ����ɣ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��С�
ʵ�� ��� | T(��) | NO��ʼŨ�� (mol��L��1) | CO��ʼŨ�� (mol��L��1) | �����ıȱ� ���(m2��g��1) |
�� | 280 | 1.2��10��3 | 5.8��10��3 | 82 |
�� |
| 1.2��10��3 |
| 124 |
�� | 350 |
|
| 82 |
(1)����ȫ���еĸ��ո�
(2)ʵ�����У�NO�����ʵ���Ũ��(c)��ʱ��(t)�ı仯��ͼ��ʾ�����ڸ���������ͼ�л���ʵ����������NO�����ʵ���Ũ��(c)��ʱ��(t)�仯�����ߣ������������ߵ�ʵ���š�

�±���Ԫ�����ڱ�����Ԫ�ص�һ���֣�������Ԫ��X����������ϼ��ǣ�5��Y�ĵ���
���ڿ�����ȼ�ա�
W | X | Y |
|
| Z |
��ش��������⣺
(1)Z��Ԫ�ط����� ��д��Z��ԭ�ӽṹʾ��ͼ�� ��
(2)W����������ﲻ����ˮ�����������ռ���Һ���÷�Ӧ�����ӷ���ʽΪ ��
(3)̽��ͬ����Ԫ�����ʵ�һЩ��ͬ���ɣ���ѧϰ��ѧ����Ҫ����֮һ�����±����г���H2ZO3���ֲ�ͬ��ѧ���ʵ��Ʋ⣬������д����Ӧ�Ļ�ѧ����ʽ(��ѧ����ʽ��Z��Ԫ�ط��ű�ʾ)
��� | �����Ʋ� | ��ѧ����ʽ |
ʾ�� | ������ | H2ZO3��4HI=Z����2I2��3H2O |
1 |
|
|
2 |
|
|
(4)��C��O��Y����Ԫ����ɵĻ�����COY�У�����ԭ�ӵ�����㶼����8���ӽṹ��д���û�����ĵ���ʽ�� ��
ij������Һ�п��ܺ���SO42-��SO32-��CO32-��HCO3-��NO3-��Cl����Br���е������ּ�һ�ֳ�������������(Mn��)���ֽ�������ʵ��(ÿ��ʵ�������Լ����������ģ�������ijЩ�ɷֿ���û�и���)��

��ش��������⣺
(1)����������ͼ��Ϣ��д�±�(����ȷ���IJ���)��
| �϶����ڵ����� | �϶�û�е����� | ����D | |
��ѧʽ�����ӷ��� |
|
|
| |
(2)������Һ���Ƿ���SO32-��SO42- ��������D���������ɫ�������ɳ���Dʱ�϶������ķ�Ӧ�����ӷ���ʽΪ ���γɳ���Bʱ��Ӧ�����ӷ���ʽΪ ��
(3)��Mn��Ϊ����������������ԭ������������20����Ҫȷ���������Ǻ������ӵķ����� ��