��Ŀ����
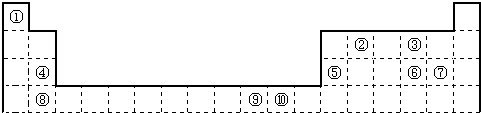
��ͼ1Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�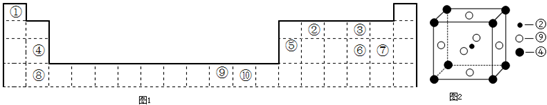
��ش��������⣺
��1����ѧ���֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص���ͼ2��ʾ��ͼ�Тڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ ���ö�Ӧ��Ԫ�ط��ű�ʾ����
��2��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ���� ��
A�������к��з��Ӽ���� B�����ں��м��Լ��ķǼ��Է���
C��ֻ����4���Ҽ���1��p-p�Ħм� D�����⻯������Т�ԭ�Ӳ���sp2�ӻ�
��3��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1����Ԫ����Ԫ�آ��γɵ�18���ӵ�X���ӵĽṹʽΪ ����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y����������Y����ͨ��ʢ�к���Ԫ�ص���������Һ�У���Ӧ�����е�ʵ������Ϊ�� ����Ӧ�����е����ӷ���ʽΪ ��
��4���ȽϢۣ��ݣ��ޣ��ߣ�������Ԫ�صĵ縺�Դ�С����С�������е�˳��Ϊ ����Ԫ�ط��ű�ʾ����
��5����Ԫ�ص��ʾ��������������壬�������ÿ����5����ԭ�ӽ��ܶ�������ԭ�Ӱ뾶Ϊd cm����ý������ܶ�Ϊ g?cm-3�����ý���ԭ�ӵ�����Ϊa g��

��ش��������⣺
��1����ѧ���֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص���ͼ2��ʾ��ͼ�Тڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ
��2��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����
A�������к��з��Ӽ���� B�����ں��м��Լ��ķǼ��Է���
C��ֻ����4���Ҽ���1��p-p�Ħм� D�����⻯������Т�ԭ�Ӳ���sp2�ӻ�
��3��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1����Ԫ����Ԫ�آ��γɵ�18���ӵ�X���ӵĽṹʽΪ
��4���ȽϢۣ��ݣ��ޣ��ߣ�������Ԫ�صĵ縺�Դ�С����С�������е�˳��Ϊ
��5����Ԫ�ص��ʾ��������������壬�������ÿ����5����ԭ�ӽ��ܶ�������ԭ�Ӱ뾶Ϊd cm����ý������ܶ�Ϊ
����������Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢޢߢ���ֱ���H��C��O��Mg��Al��S��Cl��Ca��Ni��CuԪ�أ�
��1�����þ�̯��ȷ���仯ѧʽ��
��2����ΪCԪ�أ�̼Ԫ��һ���⻯���ǵIJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־�����⻯��ΪC2H4��CԪ�ص縺�Բ������в����������Ϊ�Գƽṹ�����ڷǼ��Է��ӣ������к���1��C=C˫����4��C-H��������Ϊ�Ҽ���˫����1���Ҽ���1���м��������ӻ������Ŀȷ���ӻ���ʽ��
��3��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1��s�ܼ����������2�����ӣ�����n=2�����Ԫ�صļ۵����Ų�ʽΪ��2s22p3��ΪNԪ�أ���Ԫ����Ԫ�آ��γɵ�18���ӵ�X������N2H4����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y��Y��NH3�������İ���������ͭ��Һ�У������Ⱥ�ˮ��Ӧ����һˮ�ϰ�������ͭ��һˮ�ϰ���Ӧ����������ͭ��ɫ������������ͭ��һˮ�ϰ���Ӧ����ͭ������
��4��ͬһ����Ԫ���У�Ԫ�صĵ縺������ԭ�����������������ͬһ�����У�Ԫ�صĵ縺������ԭ���������������С��
��5�����ݦ�=
�����ܶȣ�
��1�����þ�̯��ȷ���仯ѧʽ��
��2����ΪCԪ�أ�̼Ԫ��һ���⻯���ǵIJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־�����⻯��ΪC2H4��CԪ�ص縺�Բ������в����������Ϊ�Գƽṹ�����ڷǼ��Է��ӣ������к���1��C=C˫����4��C-H��������Ϊ�Ҽ���˫����1���Ҽ���1���м��������ӻ������Ŀȷ���ӻ���ʽ��
��3��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1��s�ܼ����������2�����ӣ�����n=2�����Ԫ�صļ۵����Ų�ʽΪ��2s22p3��ΪNԪ�أ���Ԫ����Ԫ�آ��γɵ�18���ӵ�X������N2H4����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y��Y��NH3�������İ���������ͭ��Һ�У������Ⱥ�ˮ��Ӧ����һˮ�ϰ�������ͭ��һˮ�ϰ���Ӧ����������ͭ��ɫ������������ͭ��һˮ�ϰ���Ӧ����ͭ������
��4��ͬһ����Ԫ���У�Ԫ�صĵ縺������ԭ�����������������ͬһ�����У�Ԫ�صĵ縺������ԭ���������������С��
��5�����ݦ�=
| m |
| V |
����⣺����Ԫ�������ڱ��е�λ��֪���٢ڢۢܢݢޢߢ���ֱ���H��C��O��Mg��Al��S��Cl��Ca��Ni��CuԪ�أ�
��1����Ԫ��ָ���ڱ��е�λ�ÿ�֪����ΪCԪ�ء���ΪMgԪ�ء���ΪNiԪ�أ����ݾ����ṹ��֪��������Cԭ����ĿΪ1��Mgԭ����ĿΪ8��
=1��Niԭ����ĿΪ6��
=3�������ʻ�ѧʽΪ��MgNi3C���ʴ�Ϊ��MgNi3C��
��2����ΪCԪ�أ�̼Ԫ��һ���⻯���ǵIJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־�����⻯��ΪC2H4��
A��CԪ�ص縺�Բ���������ϩ�����в������������A����
B��̼��ԭ��֮����ڼ��Լ�������ϩΪ�Գƽṹ�����ڷǼ��Է��ӣ���B��ȷ��
C�������к���1��C=C˫����4��C-H��������Ϊ�Ҽ���˫����1���Ҽ���1���м�������5���Ҽ���1���м�����C����
D��Cԭ�ӳ�1��C=C˫����2��C-H�����ӻ������Ϊ3��ԭ�Ӳ���sp2�ӻ�����D��ȷ��
��ѡ��BD��
��3��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1��s�ܼ����������2�����ӣ�����n=2�����Ԫ�صļ۵����Ų�ʽΪ��2s22p3��ΪNԪ�أ���Ԫ����Ԫ�آ��γɵ�18���ӵ�X������N2H4����ṹʽΪ��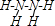 ��
��
��Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y��Y��NH3�������İ���������ͭ��Һ�У������Ⱥ�ˮ��Ӧ����һˮ�ϰ�������ͭ��һˮ�ϰ���Ӧ����������ͭ��ɫ������������ͭ��һˮ�ϰ���Ӧ����ͭ���������ӷ�Ӧ����ʽΪ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��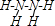 ���Ȳ�����ɫ�����������ʧ���������ɫ����Һ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
���Ȳ�����ɫ�����������ʧ���������ɫ����Һ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
��4��ͬһ����Ԫ���У�Ԫ�صĵ縺������ԭ�����������������ͬһ�����У�Ԫ�صĵ縺������ԭ���������������С�������⼸��Ԫ�ص縺�Դ�С˳����Ca��Al��S��Cl��O��
�ʴ�Ϊ��Ca��Al��S��Cl��O��
��5����Ԫ�ص��ʾ��������������壬�þ�����ͭԭ�Ӹ���=8��
+6��
=4��ͭԭ�Ӱ뾶Ϊd cm���������ⳤ=
cm=2
dcm���������=��2
dcm��3����=
=
=
g?cm-3��
�ʴ�Ϊ��
��
��1����Ԫ��ָ���ڱ��е�λ�ÿ�֪����ΪCԪ�ء���ΪMgԪ�ء���ΪNiԪ�أ����ݾ����ṹ��֪��������Cԭ����ĿΪ1��Mgԭ����ĿΪ8��
| 1 |
| 8 |
| 1 |
| 2 |
��2����ΪCԪ�أ�̼Ԫ��һ���⻯���ǵIJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־�����⻯��ΪC2H4��
A��CԪ�ص縺�Բ���������ϩ�����в������������A����
B��̼��ԭ��֮����ڼ��Լ�������ϩΪ�Գƽṹ�����ڷǼ��Է��ӣ���B��ȷ��
C�������к���1��C=C˫����4��C-H��������Ϊ�Ҽ���˫����1���Ҽ���1���м�������5���Ҽ���1���м�����C����
D��Cԭ�ӳ�1��C=C˫����2��C-H�����ӻ������Ϊ3��ԭ�Ӳ���sp2�ӻ�����D��ȷ��
��ѡ��BD��
��3��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1��s�ܼ����������2�����ӣ�����n=2�����Ԫ�صļ۵����Ų�ʽΪ��2s22p3��ΪNԪ�أ���Ԫ����Ԫ�آ��γɵ�18���ӵ�X������N2H4����ṹʽΪ��
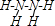 ��
����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y��Y��NH3�������İ���������ͭ��Һ�У������Ⱥ�ˮ��Ӧ����һˮ�ϰ�������ͭ��һˮ�ϰ���Ӧ����������ͭ��ɫ������������ͭ��һˮ�ϰ���Ӧ����ͭ���������ӷ�Ӧ����ʽΪ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��
 ���Ȳ�����ɫ�����������ʧ���������ɫ����Һ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
���Ȳ�����ɫ�����������ʧ���������ɫ����Һ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-����4��ͬһ����Ԫ���У�Ԫ�صĵ縺������ԭ�����������������ͬһ�����У�Ԫ�صĵ縺������ԭ���������������С�������⼸��Ԫ�ص縺�Դ�С˳����Ca��Al��S��Cl��O��
�ʴ�Ϊ��Ca��Al��S��Cl��O��
��5����Ԫ�ص��ʾ��������������壬�þ�����ͭԭ�Ӹ���=8��
| 1 |
| 8 |
| 1 |
| 2 |
|
| 2 |
| 2 |
| m |
| V |
| 4ag | ||
(2
|
| ||
| 8d3 |
�ʴ�Ϊ��
| ||
| 8d3 |
���������⿼�������ʽṹ�����ʣ��漰��ѧʽ��ȷ���������ļ��㡢�縺�Դ�С�ıȽϵ�֪ʶ�㣬ͬʱ����ѧ���ռ���������������Ԫ������������������ѵ����ܶȵļ��㣬Ҫ����ܶȹ�ʽ������ѶȽϴ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


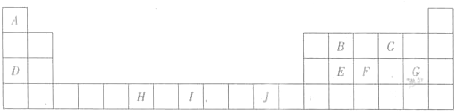
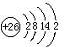
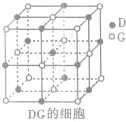 ��3��H3+������һ�����������γɽṹ���ӵ�������[HG��A2C��5]2+�γɸ�������ʱ��H3+���ӽ����������ṩ��
��3��H3+������һ�����������γɽṹ���ӵ�������[HG��A2C��5]2+�γɸ�������ʱ��H3+���ӽ����������ṩ��