��Ŀ����
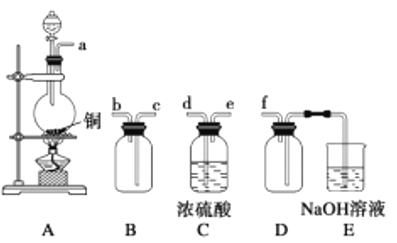
����Ŀ��������һ����Ļ�ѧ��Դ���⣬��ˮ��Դ�ۺ����õIJ�������ͼ��
![]()
(1)�ɺ�ˮ��ȡ�Ĵ����г�����![]() �ȣ����Լ���_______________(�ѧʽ)��ȥ����Ӧ�����ӷ���ʽΪ__________________��
�ȣ����Լ���_______________(�ѧʽ)��ȥ����Ӧ�����ӷ���ʽΪ__________________��
(2)����ٷ�Ӧ�����ӷ���ʽΪ__________________��
(3)��������嵥�ʱ�SO2��ԭΪ![]() ����Ԫ�ر�����Ϊ
����Ԫ�ر�����Ϊ![]() ���Ӹ÷�Ӧԭ���в��ܵó���ķǽ�����ǿ������ԭ����__________________������0.25 mol SO2��������ת�Ƶĵ��ӵ����ʵ���Ϊ__________mol��
���Ӹ÷�Ӧԭ���в��ܵó���ķǽ�����ǿ������ԭ����__________________������0.25 mol SO2��������ת�Ƶĵ��ӵ����ʵ���Ϊ__________mol��
���𰸡�BaCl2 ![]()
![]() SO2��Br2�ķ�Ӧ���ǵ���֮����û���Ӧ,���,���ܵó���ķǽ����Ա���ǿ 0.5
SO2��Br2�ķ�Ӧ���ǵ���֮����û���Ӧ,���,���ܵó���ķǽ����Ա���ǿ 0.5
��������
��1�����ӵ�ԭ��Ϊ�������µ����ʣ��ײ�����������Ŀ����
��2�������Ϊ�����������������嵥�ʺ������ӵķ�Ӧ��
��3������֮����û���Ӧ���ԱȽϷǽ����Ե�ǿ�������õ�ʧ�����غ����ת�Ƶ�������
��1)��ȥ![]() ���������µ����ʣ�Ӧ��ѡ��BaCl2����Ӧ�����ӷ���ʽΪ��
���������µ����ʣ�Ӧ��ѡ��BaCl2����Ӧ�����ӷ���ʽΪ��![]() ��
��
�ʴ�Ϊ��BaCl2��![]() ��
��
��2)�������ͨ������ʱ������������������������Ӧ�����ӷ���ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
(3)SO2��Br2�ķ�Ӧ���ǵ���֮����û���Ӧ����ˣ����ܵó���ķǽ����Ա���ǿ����SO2��Ӧʱ������+4�����ߵ�+6�ۣ�����0.25molSO2ת�Ƶĵ���Ϊ![]() ��
��
�ʴ�Ϊ��SO2��Br2�ķ�Ӧ���ǵ���֮����û���Ӧ,���,���ܵó���ķǽ����Ա���ǿ��0.5��

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�