��Ŀ����
��1����֪����Mg(s)+Cl2(g)=MgCl2(s) ����=��641kJ��mol��1��
��1/2Ti(s)+ Cl2(g) =1/2TiCl4(1) ���� ����=��385 kJ��mol��1��
��2Mg(s)��TiCl4(1)��2MgCl2(s)��Ti(s)�ķ�Ӧ��Ϊ����= ��
��2����֪�״���CH3OH���ڳ�����ΪҺ�壬����ֵΪ22.7kJ?g-1����״��ı�ȼ���ȵ��Ȼ�ѧ����ʽΪ_______ _____ __��
��3�����״�������������������Һ��Ƴ�ȼ�ϵ�أ��õ��
������ӦʽΪ__________________ ______________��
��4����ͼΪ��ҵ���ȼҵ�ĵ���ʾ��ͼ����ͼ�ش�
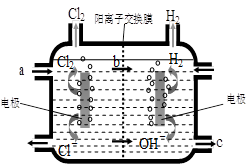
�ٸõ����з�����Ӧ�������ӷ�Ӧ����ʽΪ
����û�������ӽ���Ĥ������һ��ʱ����ڵ��۵���Һ�п��ܷ����Ļ�ѧ��Ӧ����ʽΪ______ ____________________��
��1/2Ti(s)+ Cl2(g) =1/2TiCl4(1) ���� ����=��385 kJ��mol��1��
��2Mg(s)��TiCl4(1)��2MgCl2(s)��Ti(s)�ķ�Ӧ��Ϊ����= ��
��2����֪�״���CH3OH���ڳ�����ΪҺ�壬����ֵΪ22.7kJ?g-1����״��ı�ȼ���ȵ��Ȼ�ѧ����ʽΪ_______ _____ __��
��3�����״�������������������Һ��Ƴ�ȼ�ϵ�أ��õ��
������ӦʽΪ__________________ ______________��
��4����ͼΪ��ҵ���ȼҵ�ĵ���ʾ��ͼ����ͼ�ش�

�ٸõ����з�����Ӧ�������ӷ�Ӧ����ʽΪ
����û�������ӽ���Ĥ������һ��ʱ����ڵ��۵���Һ�п��ܷ����Ļ�ѧ��Ӧ����ʽΪ______ ____________________��
(1)-512 kJ?mol��1
(2)CH3OH(l)��3 2O2(g)��CO2(g)��2H2O(l) ��H��726.4kJ?mol��1
(3)O2��4e����2H2O��4OH��
(4)��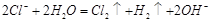
��Cl2+2NaOH ="NaClO" +NaCl+H2O
(2)CH3OH(l)��3 2O2(g)��CO2(g)��2H2O(l) ��H��726.4kJ?mol��1
(3)O2��4e����2H2O��4OH��
(4)��
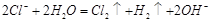
��Cl2+2NaOH ="NaClO" +NaCl+H2O
���������(1)����=�١�2���ڡ�2��-512 kJ?mol��1����2����ֵ��ָ����1ǧ�ˣ�ÿ�����ף�ij�ֹ��壨���壩ȼ����ȫȼ�շų�����������1mol�״�ȼ�շų�������Ϊ22.7kJ?g-1��1mol��32g/mol��726.4kJ���״�ȼ��Ӧ�����ȶ���CO2(g)��H2O(l)����3��ȼ�ϵ������������ԭ��Ӧ���������ڷŵ磻��4���ȼҵ����Ҫ�Ļ�����������ⱥ��NaCl��Һ������Ϊ�������ڷŵ磬����Ϊ�����ӣ���ˮ����ģ��ڷŵ磻��û�������ӽ���Ĥ�����������Ե�������ұߣ����������Ʒ���������ԭ��Ӧ��
��������Ӧ�Ⱥ͵���������߿��ij���֪ʶ�㣬����Ӧע��������Ŀ��顣ѧ�Ƶ��ۺϿ�����ǽ���߿������ƣ�����ֵ���ۺϿ��飬�ѶȽϴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
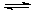 H++ SO42-
H++ SO42- �ķ�ˮ�ᷢ�����·�Ӧ��
�ķ�ˮ�ᷢ�����·�Ӧ��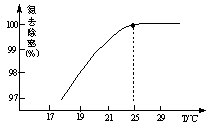
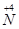 ��
��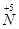 ��ת�����������һ�������Խ��� ��
��ת�����������һ�������Խ��� �� 2CO2+N2��
2CO2+N2��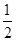 O2(g)=CO2(g) ��H=-283.0kJ��mol-1
O2(g)=CO2(g) ��H=-283.0kJ��mol-1 C(CO)/mol��L-1
C(CO)/mol��L-1
 2.70��10-3
2.70��10-3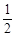 O2(g)��ZnO(s) ��H ����351.1kJ��mol��1
O2(g)��ZnO(s) ��H ����351.1kJ��mol��1 2NH3�������仯��ͼ��ʾ��
2NH3�������仯��ͼ��ʾ��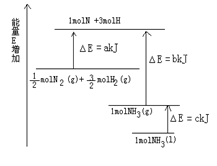
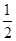 N2(g)+
N2(g)+ H2(g)
H2(g)  2NO (g)���÷�Ӧ�ǵ�������β���к���NO��ԭ��֮һ��
2NO (g)���÷�Ӧ�ǵ�������β���к���NO��ԭ��֮һ��
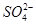 (aq)+
(aq)+ (aq)+2
(aq)+2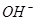 (aq) = BaSO4(s)+2H
(aq) = BaSO4(s)+2H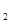 O(l);
O(l);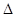 H =
H =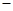 57.3 kJ/mol
57.3 kJ/mol H
H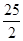 O
O