��Ŀ����
����Ŀ����ͭ�����Ҫ�ɷ���Cu2S�����϶��Fe2O3��SiO2���ʣ����̿����Ҫ�ɷ���MnO2�����н϶��SiO2���ʡ���ͭ���ʪ��ұ����Ŀǰ�����ⶼ����̽��֮�С���������ԭ�����뷨��һ�ֽ�Ϊ�����ʪ��ұ�������������������£�

�ش��������⣺
(1)��������ʱ��Ϊ��߿�ʯ�Ľ����ʣ����д�ʩ�ɲ�ȡ����________��(����)
A �ʵ���߽����¶� B �ʵ��ӳ�����ʱ�� C �����ʯ������
(2)��100g��ͭ���40g 98����Ũ�����ϣ���80��90�������½���2.5h��ͭ�Ľ����������̿������ı仯���£�
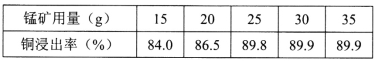
�����100g��ͭ�����̿������������Ϊ_____g��
(3)����������Ľ���Һ�к���CuSO4��MnSO4��Fe2(SO4)3�����ʣ�������I���к��е���ɫ���嵥�ʣ�������ʱ��������ɫ����Ļ�ѧ����ʽΪ__________________________��
(4)�о�����������Fe2O3�ڡ���������Ӧ��������Ҫ��ý�����ã��ٽ�Cu2S��MnO2���ܽ⣬�䷴Ӧ�������£�
��Fe2O3+3H2SO4=Fe2(SO4)3+3H2O��
��Cu2S+2Fe2(SO4)3=2CUSO4+4FeSO4+S����
��___________________________________��(д����Ӧ�Ļ�ѧ����ʽ)
(5)������II������Ҫ�ɷ���______________�������̡��õ�������ɫ��Һ�г�NH4+��H+�⣬�����е�������Ϊ__________��(д��ѧʽ)
(6)����ҺIII�����ᾧ�ɵõ�(NH4)2SO4���塣�ᾧʱ����________ʱ������ֹͣ���ȡ�
���𰸡�AB 25 2MnO2+Cu2S+4H2SO4==S+2CuSO4+2MnSO4+4H2O 2FeSO4+MnO2+2H2SO4==MnSO4+Fe2(SO4)3+2H2O Fe(OH)3 [Cu(NH3)4]2+ ��Һ������־�Ĥ(����Һ���־���)
��������
��ͭ�����̿����ʱ����������ͭ���������������̵Ļ��Һ����������������衢������������IΪ�������衢S������̼����淋���pH��������������������������̼���壬������IIΪ��������������̼����李�������̼���̡�
(1)A�ʵ���߽����¶ȣ���Ӧ���ʼӿ죬��������ߣ�A�������⣻
B�ʵ��ӳ�����ʱ�䣬ʹ��۳������Ӵ�����߽����ʣ�B�������⣻
C�����ʯ�����ȣ����ٽӴ�����������ʽ��ͣ�C�����ⲻ����
��ΪAB��
(2)���ݱ������ݣ�����25g���̿�ʱ��ͭ�Ľ����ʼ�Ϊ89.9%�������ӽ����ʲ�����ߣ���ѡ��25gΪ�ˣ�
(3)����ͭ������������������������������̡�����ͭ������ɫ�ij������ˮ������ʽΪ2MnO2+Cu2S+4H2SO4==S+2CuSO4+2MnSO4+4H2O��
(4)������������������̡��������������������������̺�ˮ������ʽΪ2FeSO4+MnO2+2H2SO4==MnSO4+Fe2(SO4)3+2H2O��
(5)������֪��������II������Ҫ�ɷ����������������������õ�������ɫ��Һ�г�NH4+��H+�⣬��Һ�е�ͭ�������백�γɵ������ӵ���ʽ���ڣ���[Cu(NH3)4]2+��
(6)����ҺIII�����ᾧ�ɵõ�(NH4)2SO4���塣����������ֽ⣬������Һ�г��־���ʱֹͣ���ȡ�