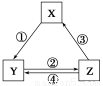
ΧβΡΩΡΎ»ί
AΓΔBΓΔCΓΔDΓΔEΈε÷÷‘ΣΥΊΘ§A‘ΣΥΊΒΡ÷ήΤΎ ΐΓΔ÷ςΉε ΐΓΔ‘≠Ή”–ρ ΐœύΆ§ΘΜBΒΡΜυΧ§‘≠Ή”ΚΥΆβ”–3÷÷ΡήΝΩ≤ΜΆ§ΒΡ‘≠Ή”ΙλΒάΘ§«“ΟΩ÷÷ΙλΒά÷–ΒΡΒγΉ” ΐœύΆ§ΘΜC‘ΣΥΊΒΡΒγάκΡή»γΆΦΥυ ΨΘΜD‘ΣΥΊΒΡΦέΒγΉ”ΙΙ–ΆΈΣnsnnpnΘΪ2ΘΜE «ΒΎ4÷ήΤΎΒΡΙΐΕ…‘ΣΥΊΘ§―ΣΚλΒΑΑΉ÷–ΒΡE‘ΣΥΊ”κBD–Έ≥…ΒΡ≈δΈΜΦϋ±»”κD2–Έ≥…ΒΡ≈δΈΜΦϋ«ΩΓΘEΒΞ÷ ”κBD–Έ≥…ΒΡ≈δΚœΈοEΘ®BDΘ©5Θ§≥ΘΈ¬œ¬≥ “ΚΧ§Θ§»έΒψΈΣΘ≠20.5ΓφΘ§Ζ–ΒψΈΣ103 ΓφΘ§“Ή»ή”ΎΖ«ΦΪ–‘»ήΦΝΓΘ
C‘ΣΥΊΒΡΒγάκΡή
Θ®1Θ©EΘ®BDΘ©5ΨßΧε τ”Ύ________Θ®ΧνΨßΧεάύ–ΆΘ©ΓΘ
Θ®2Θ©A‘ΣΥΊΚΆB‘ΣΥΊΉι≥…ΒΡΜ·ΚœΈοΖ÷Ή”÷°Φδ________Θ®ΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±Θ©–Έ≥…«βΦϋΓΘ
Θ®3Θ©ΜυΧ§E‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ___________________________________ ΓΘ
Θ®4Θ©BΓΔCΓΔD»ΐ÷÷‘ΣΥΊΒΡΒγΗΚ–‘”…¥σΒΫ–ΓΒΡΥ≥–ρ «________Θ®Χν‘ΣΥΊΖϊΚ≈Θ©ΓΘ
Θ®5Θ©C2ΚΆB2A2ΒΡΖ÷Ή”÷–ΗυΨίΒγΉ”‘Τ÷ΊΒΰΒΡΖΫ Ϋ≤ΜΆ§Θ§ΕΦΑϋΚ§ΒΡΙ≤ΦέΦϋάύ–Ά”–________ΓΘ
Θ®6Θ©“―÷Σ‘≠Ή” ΐΚΆΒγΉ” ΐœύΆ§ΒΡΈΔΝΘΫ–Β»ΒγΉ”ΧεΘ§Β»ΒγΉ”ΧεΒΡΫαΙΙœύΥΤΓΘΗυΨί
œ¬±μ ΐΨίΘ§ΥΒΟςBDΖ÷Ή”±»C2Ζ÷Ή”ΜνΤΟΒΡ‘≠“ρ_____________________________ΓΘ
| XΓΣY | XΘΫY | XΓ‘Y |
BDΒΡΦϋΡή/kJΓΛmolΘ≠1 | 357.7 | 798.9 | 1 071.9 |
C2ΒΡΦϋΡή/kJΓΛmolΘ≠1 | 154.8 | 418.4 | 941.7 |
Θ®1Θ©Ζ÷Ή”ΨßΧε
Θ®2Θ©≤ΜΡή
Θ®3Θ©1s22s22p63s23p63d64s2
Θ®4Θ©OΓΔNΓΔC
Θ®5Θ©Π“ΦϋΚΆΠ–Φϋ
Θ®6Θ©CO÷–ΕœΝ―ΒΎ“ΜΗωΠ–ΦϋœϊΚΡΒΡΡήΝΩΘ®273 kJΘ©±»N2÷–ΕœΝ―ΒΎ“ΜΗωΠ–ΦϋœϊΚΡΒΡΡήΝΩΘ®523.3 kJΘ©–ΓΘ§COΒΡΒΎ“ΜΗωΠ–ΦϋΫœ»ί“ΉΕœΝ―Θ§“ρ¥ΥCOΫœΜνΤΟ
ΓΨΫβΈωΓΩ”…Χβ÷––≈œΔΩ…ΆΤ÷ΣA «HΓΔB «CΓΔC «NΓΔDΈΣOΓΔEΈΣFeΓΘΘ®1Θ©≈δΚœΈοFeΘ®COΘ©5≥ΘΈ¬œ¬≥ “ΚΧ§Θ§“Ή»ή”ΎΖ«ΦΪ–‘»ήΦΝΘ§‘ρFeΘ®COΘ©5ΨßΧεΈΣΖ÷Ή”ΨßΧεΓΘΘ®2Θ©C‘ΣΥΊΒΡΖ«Ϋπ τ–‘Ϋœ»θΘ§Ι CΓΔH‘ΣΥΊ–Έ≥…ΒΡΜ·ΚœΈο÷–ΟΜ”–«βΦϋΓΘΘ®3Θ©¬‘ΓΘΘ®4Θ©CΓΔNΓΔO «Ά§÷ήΤΎΒΡ‘ΣΥΊΘ§ΤδΒγΗΚ–‘ΥφΉ≈‘≠Ή”–ρ ΐΒΡ‘ω¥σΕχ‘ω¥σΘ§Ι »ΐ’ΏΒγΗΚ–‘”…¥σΒΫ–ΓΒΡΥ≥–ρ «ΘΚOΓΔNΓΔCΓΘΘ®5Θ©N2”κC2H2ΒΡΖ÷Ή”÷–ΕΦΑϋΚ§ΒΡΙ≤ΦέΦϋ”–Π“ΦϋΚΆΠ–ΦϋΘ®‘ΎNΓ‘NΚΆCΓ‘C÷–ΕΦΑϋΚ§ΝΥ“ΜΧθΠ“ΦϋΚΆΝΫΧθΠ–ΦϋΘ©ΓΘΘ®6Θ©CO”κN2 «Β»ΒγΉ”ΧεΘ§ΫαΙΙœύΥΤΘ§ΒΪCO÷–ΕœΝ―ΒΎ“ΜΗωΠ–ΦϋœϊΚΡΒΡΡήΝΩΈΣΘΚ1 071.9Θ≠798.9ΘΫ273 kJΘΜΕχN2÷–ΕœΝ―ΒΎ“ΜΗωΠ–ΦϋœϊΚΡΒΡΡήΝΩΈΣΘΚ941.7Θ≠418.4ΘΫ523.3 kJΘ§Ω…ΦϊCOΒΡΒΎ“ΜΗωΠ–Φϋ»ί“ΉΕœΝ―Θ§“ρ¥ΥCO±»N2ΜνΤΟΓΘ

 ΜΤΗ‘Ψ≠Βδ»ΛΈΕΩΈΧΟœΒΝ–¥πΑΗ
ΜΤΗ‘Ψ≠Βδ»ΛΈΕΩΈΧΟœΒΝ–¥πΑΗ ΤτΕΪ–ΓΧβΉς“Β±ΨœΒΝ–¥πΑΗ
ΤτΕΪ–ΓΧβΉς“Β±ΨœΒΝ–¥πΑΗΒΣΓΔΝΉΓΔ…ι «Ά§Ήε‘ΣΥΊΘ§ΗΟΉε‘ΣΥΊΒΞ÷ ΦΑΤδΜ·ΚœΈο‘Ύ≈©“©ΓΔΜ·Ζ …ζ≤ζΒ»ΖΫΟφ”–÷Ί“Σ”Π”ΟΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
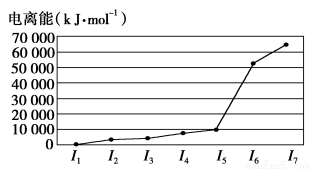
Θ®1Θ©…ι‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ_____________________________________ΓΘ
Θ®2Θ©K3[FeΘ®CNΘ©6]ΨßΧε÷–Fe3ΘΪ”κCNΘ≠÷°ΦδΒΡΦϋ–ΆΈΣ________Θ§ΗΟΜ·―ßΦϋΡήΙΜ–Έ≥…ΒΡ‘≠“ρ «______________________________________________________ΓΘ
Θ®3Θ©NH4ΘΪ÷–ΒΣ‘≠Ή”ΒΡ‘”Μ·άύ–ΆΈΣ________Θ§NH4ΘΪΒΡΩ’ΦδΙΙ–ΆΈΣ________ΓΘ
Θ®4Θ©“―÷ΣΘΚ
| CH4 | SiH4 | NH3 | PH3 |
Ζ–ΒψΘ®KΘ© | 101.7 | 161.2 | 239.7 | 185.4 |
Ζ÷ΫβΈ¬Ε»Θ®KΘ© | 873 | 773 | 1 073 | 713.2 |
Ζ÷Έω…œ±μ÷–ΥΡ÷÷Έο÷ ΒΡœύΙΊ ΐΨίΘ§«κΜΊ¥πΘΚ
CH4ΚΆSiH4±»ΫœΘ§NH3ΚΆPH3±»ΫœΘ§Ζ–ΒψΗΏΒΆΒΡ‘≠“ρ «___________________________________________________________________________ΓΘ
CH4ΚΆSiH4±»ΫœΘ§NH3ΚΆPH3±»ΫœΘ§Ζ÷ΫβΈ¬Ε»ΗΏΒΆΒΡ‘≠“ρ «_________________________________________________________________________ΓΘ
ΫαΚœ…œ ω ΐΨίΚΆΙφ¬…≈–ΕœΘ§“ΜΕ®―Ι«Ωœ¬HFΚΆHClΒΡΜλΚœΤχΧεΫΒΈ¬ ±________œ»“ΚΜ·ΓΘ
Θ®5Θ©ΒγΗΚ–‘Θ®”ΟX±μ ΨΘ©“≤ «‘ΣΥΊΒΡ“Μ÷÷÷Ί“Σ–‘÷ Θ§œ¬±μΗχ≥ω8÷÷‘ΣΥΊΒΡΒγΗΚ–‘ ΐ÷ΒΘΚ
‘ΣΥΊ | Na | Mg | Al | Si | P | S | Cl | K |
ΒγΗΚ–‘ | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 | 0.8 |
«κΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
ΙάΦΤΗΤ‘ΣΥΊΒΡΒγΗΚ–‘ΒΡ»Γ÷ΒΖΕΈßΘΚ________<X<________ΓΘΨ≠―ιΙφ¬…ΗφΥΏΈ“Ο«ΘΚΒ±–Έ≥…Μ·―ßΦϋΒΡΝΫ‘≠Ή”œύ”Π‘ΣΥΊΒΡΒγΗΚ–‘≤ν÷Β¥σ”Ύ1.7 ±Θ§Υυ–Έ≥…ΒΡ“ΜΑψΈΣάκΉ”ΦϋΘΜΒ±–Γ”Ύ1.7 ±Θ§“ΜΑψΈΣΙ≤ΦέΦϋΓΘ ‘ΆΤΕœAlCl3÷––Έ≥…ΒΡΜ·―ßΦϋΒΡάύ–ΆΦΑΤδάμ”…ΘΚ__________________________ΓΘ