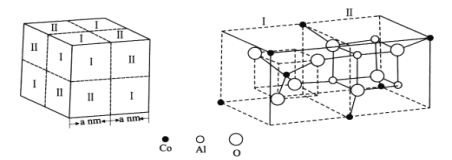
��Ŀ����
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ������84����Һ��ͨ��ϡ�͵�100��(���֮��)��ʹ�á���ش��������⣺
��1������84����Һ�������ʵ���Ũ��ԼΪ______mol��L-1��
84����Һ
��Ч�ɷ֣�NaClO
���1000 mL
����������25%
�ܶȣ�1.19 g��cm-3
��2����ͬѧ���ĸ���84����Һ�����䷽������NaClO��������480 mL�����ʵ���Ũ�ȵ�����Һ������˵����ȷ����_____(����ĸ)��

A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɺ��������������Һ
C�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
D����Ҫ����NaClO���������Ϊ143.0g
��3����84����Һ����ϡ�����ϻ����һ�ֻ���ɫ�����壬д����Ӧ�����ӷ���ʽ��_____
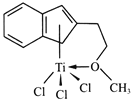
��4����������Ҫ�ɷ�ΪFeTiO3��TiΪ+4�ۣ��������������������Ҫ��TiOCl42����ʽ���ڣ�д����Ӧ�����ӷ���ʽ��_______
���𰸡�4 C ClO-+Cl-+2H+=Cl2����H2O FeTiO3+4H++4Cl=Fe2++TiOCl42+2H2O
��������
(1)����������Ũ�ȵĻ��㣬���ù�ʽ![]() ��
��
(2)����һ�����ʵ���Ũ�����Ƶ�ʵ�����̷����жϣ�
(3)NaClO��HCl�Ĺ��з�Ӧ������������
(4)�����غ㷨���
(1)����������Ũ�ȵĻ��㣬���ù�ʽ![]() ����������
����������![]() ��
��
(2)A����NaClO��������һ�����ʵ���Ũ�ȣ�����ҪԲ����ƿ�ͷ�Һ©����2�ֲ���Ҫ��A�����
B������ƿ������ˮϴ�Ӻ�����ƿ���ܺ�ɣ���������ƿ����ˮ����Ũ��û��Ӱ�죬B�����
C�����ƹ����У�δ������ˮϴ���ձ��Ͳ����������²�������δת������ƿ��������Һƫ�ͣ�C����ȷ��
D������480mL����Һ������ʵ������ֻ��500mL������ƿ����Ҫ����500mL���ܼ����㡣![]() ��D�����
��D�����
�����ѡC��
(3)NaClO��HCl��Ӧ��Ϊ���з�Ӧ������������ClO����Cl����2H��=Cl2����H2O��
(4)��������Ҫ�ɷ�ΪFeTiO3������Ti�Ļ��ϼ�Ϊ+4����Fe�Ļ�����Ϊ+2�������ᡰ�����������Ҫ��TiOCl42�����ڣ��仯�ϼ�û�б䣬�Ʋ��䲻��������ԭ��Ӧ�����ݵ���غ��ԭ���غ㣬��ѧ����ʽΪΪFeTiO3+4H++4Cl=Fe2++TiOCl42+2H2O��