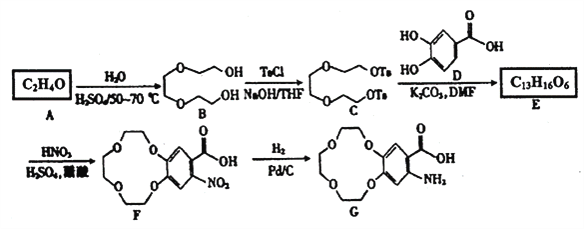
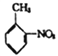
ЬтФПФкШн
ЁОЬтФПЁПМюЪНбѕЛЏФј(NiOOH)ПЩгУзїФјЧтЕчГиЕФе§МЋВФСЯЃЌПЩгУЗЯФјДпЛЏМСЃЈжївЊКЌNiЁЂA1ЃЌЩйСПCrЁЂFeSЕШ)РДжЦБИЃЌЦфЙЄвеСїГЬШчЯТЃК
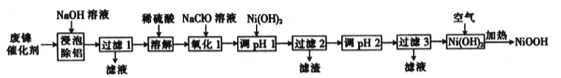
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЁАНўХнГ§ТСЁБЪБЃЌЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________________ЁЃ
ЃЈ2ЃЉЁАШмНтЁБЪБЗХГіЕФЦјЬхЮЊ_________ЃЈЬюЛЏбЇЪН)ЁЃ
ЃЈ3ЃЉЁАбѕЛЏ1ЁБЪБЃЌЫсадЬѕМўЯТЃЌШмвКжаЕФFe2+БЛбѕЛЏЮЊFe3+ЃЌЦфРызгЗНГЬЪНЮЊ______________ЁЃ
ЃЈ4ЃЉМКжЊИУЬѕМўЯТН№ЪєРызгПЊЪМГСЕэКЭЭъШЋГСЕэЕФpHШчЯТБэЃК
ПЊЪМГСЕэЕФpH | ЭъШЋГСЕэЕФpH | |
Ni2+ | 6.2 | 8.6 |
Fe2+ | 7.6 | 9.1 |
Fe3+ | 2.3 | 3.3 |
Cr3+ | 4.5 | 5.6 |
ЁАЕїpH 1ЁБЪБЃЌШмвКpHЗЖЮЇЮЊ__________ЃЛЙ§ТЫ2ЫљЕУТЫдќЕФГЩЗжЮЊ___________(ЬюЛЏбЇЪН)ЁЃ
ЃЈ5ЃЉаДГідкПеЦјжаМгШШNi(OH)2жЦШЁNiOOHЕФЛЏбЇЗНГЬЪН______________ЁЃ
ЃЈ6ЃЉШєМгШШВЛГфЗжЃЌдђжЦЕУЕФNiOOHжаЛсЛьгаNi(OH)2ЃЌЦфзщГЩПЩБэЪОЮЊxNiOOHЁЄy Ni(OH)2ЁЃЯжГЦШЁ8.29g xNiOOHЁЄy Ni(OH)2бљЦЗШмгкЯЁСђЫсЃЌНСАшжСШмвКГЮЧхЃЌЖЈШнжС200mL,ДгжавЦШЁ20.00 mL,гУ0.010molЁЄL-1ЕФKMnO4БъзМШмвКЕЮЖЈЃЌжиИДЩЯЪіВйзї2ДЮЃЌЦНОљЯћКФKMnO4 БъзМШмвК 20.00 mLЁЃвбжЊ 5Ni2++MnO4-+8H+=5Ni3++Mn2++4H2OЃЌдђx=_________ЃЌy=_________ЁЃ
ЁОД№АИЁП 2Al+2NaOH+2H2O=2NaAlO2+3H2Ёќ H2КЭ H2S 2Fe2++ClO-+2H+=2Fe3++Cl-+H2O 5.66.2 Cr(OH)3КЭ Fe(OH)3 4Ni(OH)2+O2![]() 4NiOOH+2H2O 8 1
4NiOOH+2H2O 8 1
ЁОНтЮіЁП(1)ЁАНўХнГ§ТСЁБЪБЃЌЧтбѕЛЏФЦНЋТСШмНтЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2Al+2NaOH+2H2O=2NaAlO2+3H2ЁќЃЌЙЪД№АИЮЊЃК2Al+2NaOH+2H2O=2NaAlO2+3H2ЁќЃЛ
(2)ЁАШмНтЁБЪБЃЌСђЫсгыNiЁЂCrЁЂFeSЗДгІЃЌЗХГіЕФЦјЬхЮЊH2КЭ H2SЃЌЙЪД№АИЮЊЃКH2КЭ H2SЃЛ
(3)ЁАбѕЛЏ1ЁБЪБЃЌЫсадЬѕМўЯТЃЌДЮТШЫсФЦНЋШмвКжаЕФFe2+БЛбѕЛЏЮЊFe3+ЃЌРызгЗНГЬЪНЮЊ2Fe2++ClO-+2H+=2Fe3++Cl-+H2OЃЌЙЪД№АИЮЊЃК2Fe2++ClO-+2H+=2Fe3++Cl-+H2OЃЛ
(4)ИљОнН№ЪєРызгПЊЪМГСЕэКЭЭъШЋГСЕэЕФpHБэЃЌЁАЕїpH 1ЁБЪБЃЌФПЕФЪЧНЋFe3+КЭCr3+Г§ШЅЃЌвђДЫШмвКpHЗЖЮЇЮЊ5.66.2ЃЌЙ§ТЫ2ЫљЕУТЫдќЕФГЩЗжЮЊCr(OH)3КЭ Fe(OH)3ЃЌЙЪД№АИЮЊЃК5.66.2ЃЛCr(OH)3КЭ Fe(OH)3ЃЛ
(5)дкПеЦјжаМгШШNi(OH)2жЦШЁNiOOHЕФЛЏбЇЗНГЬЪНЮЊ4Ni(OH)2+O2![]() 4NiOOH+2H2OЃЌЙЪД№АИЮЊЃК4Ni(OH)2+O2
4NiOOH+2H2OЃЌЙЪД№АИЮЊЃК4Ni(OH)2+O2![]() 4NiOOH+2H2OЃЛ
4NiOOH+2H2OЃЛ
(6)ЯћКФKMnO4ЮяжЪЕФСПЃК0.01 molL-1ЁС0.02L=2ЁС10-4 molЃЌИљОн5Ni2++MnO4-+8H+=5Ni3++Mn2++4H2OПЩжЊЃЌбљЦЗжаКЌгаЕФNi2+ЮяжЪЕФСПЃК2ЁС10-4 molЁС5ЁС(200ЁТ20)=0.01 molЃЌ0.01 molЕФNi(OH)2ЕФжЪСПЮЊ93g/molЁС0.01 mol=0.93gЃЌдђNiOOHЕФжЪСПЮЊ8.29g-0.93g=7.36gЃЌЮяжЪЕФСПЮЊ![]() =0.08molЃЌxЃКy=n(NiOOH)ЃКn[Ni(OH)2]=0.08 molЃК0.01 mol=8ЃК1ЃЌдђx=8ЃЌy=1ЃЌЙЪД№АИЮЊЃК8ЃЛ1ЁЃ
=0.08molЃЌxЃКy=n(NiOOH)ЃКn[Ni(OH)2]=0.08 molЃК0.01 mol=8ЃК1ЃЌдђx=8ЃЌy=1ЃЌЙЪД№АИЮЊЃК8ЃЛ1ЁЃ