��Ŀ����
����Ŀ��������������(Na2S2O4)��ӡȾ��ҵ��һ�ֳ���ԭ�ϣ��������������ֳƱ��շۣ�������ˮ���������ڼ״�����������ȡ���ˮ���ᷢ����Ӧ�ų��������ȣ���������ȼ�գ���ҵ�Ʊ�������ͼ��
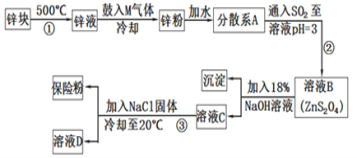
��ش��������⣺
��1�����������������Ƶ�ע������___(��дһ��)��
��2�����������MΪ������һ����Ҫ�ɷ֣���M�ĵ���ʽΪ___��
��3�����̢ڵĻ�ѧ����ʽΪ__��
��4�����̢۵ķ��뷽������Ϊ���ˡ�ϴ�ӡ��������ϴ�������Լ���___��ʵ��ʱ����NaCl�����������__��
��5�������������ƿ������ڳ�ȥ��ˮ�е��ظ��������(Cr2O72-��ת��ΪCr3+��S2O42-��ת��ΪSO42-)��д���÷�Ӧ�����ӷ���ʽ___��
���𰸡��ܷ⡢���������� ![]() Zn+2SO2=ZnS2O4 �״� ����Na2S2O4���ܽ�Ȼ�����Na+Ũ�ȣ�����Na2S2O4�ᾧ���� Cr2O72-+S2O42-+6H+=2Cr3++2SO42- +3H2O
Zn+2SO2=ZnS2O4 �״� ����Na2S2O4���ܽ�Ȼ�����Na+Ũ�ȣ�����Na2S2O4�ᾧ���� Cr2O72-+S2O42-+6H+=2Cr3++2SO42- +3H2O
��������
п������ڻ�����пҺ�й���M���壬ʹҺ̬п��������ȴ�õ�п�ۣ���ˮ�γɷ�ɢϵ����ͨ���������Ӧ�õ�ZnS2O4������NaOH��Һ��Ӧ�õ�������п������Na2S2O4��������NaCl����Na2S2O4���ܽ�ȣ�����Na2S2O4����ҺD�к���NaCl���ݴ˷�������
��1����Ϊ��Ŀ�й��ڱ��շ�˵��Ϊ����������ȡ���ˮ���ᷢ����Ӧ�ų��������ȣ���������ȼ�ա����Ա���ʱӦ���ܷⱣ�棬�������ȣ�ͬʱҲӦ����������ȼ����룻
��2�����������MΪ������һ����Ҫ�ɷ֣���Mֻ���ǵ����������ʽΪ![]() ��
��
��3�����̢ڵ�Ŀ���ǽ�����пת��ΪZnS2O4��������Zn����ͨ��SO2���仯ѧ����ʽΪ��Zn+2SO2 = ZnS2O4��
��4��ϴ�Ӳ�Ʒʱ�����ǵ���Ŀ�ж��ڱ��շ۵�������������ˮ���������ڼ״��������ü״�ϴ�ӿ��Է�ֹ��Ʒ����ʧ��ʵ���м����Ȼ��Ƶ�Ŀ��������ͬ����ЧӦ������Na2S2O4���ܽ�ȣ��Ա������������ʴ�Ϊ���״�������Na2S2O4���ܽ�Ȼ�����Na+Ũ�ȣ�����Na2S2O4�ᾧ������
��5�������������ƿ������ڳ�ȥ��ˮ�е��ظ��������(Cr2O72-��ת��ΪCr3+��S2O42-��ת��ΪSO42-)�����ϵõ����ַ�Ӧ��Cr2O72-+S2O42- �� Cr3+ +SO42-��Cr2O72-���ϼ۽���6�ۣ�2��Cr��ÿ������3�ۣ���S2O42-���ϼ�����6�ۣ�2��S��ÿ������3�ۣ��������������ӵ�ϵ������1������ʽ��ΪCr2O72-+S2O42- �� 2Cr3+ + 2SO42-���ٸ���ԭ�Ӹ����غ�͵���غ�õ���Cr2O72-+S2O42-+6H+ = 2Cr3+ +2SO42- +3H2O��

����Ŀ����Ԫ���Ƕ�ֲ����������ȱ�ٵ�Ԫ�أ��㷺��������Ȼ���С�
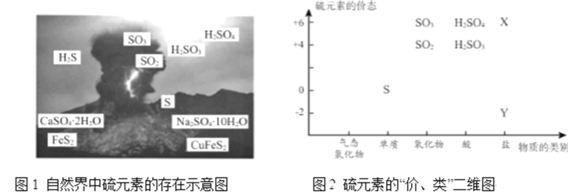
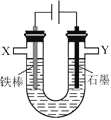
��ͼ1��ѡ�����ͼ2Ҫ��� X ��Y ���������ʣ�X ______��Y ________��
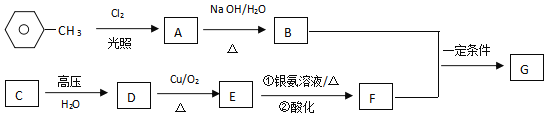
(2)��������Ҫ�Ļ���ԭ�ϣ�С��ͬѧ������Ĺ�ҵ�Ʊ������ʽ���̽�����������ϣ���ҵ������Ĺ������£�
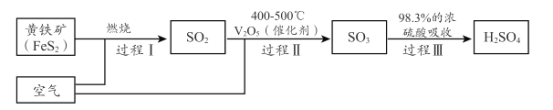
��������ҵ����������У�û�з���������ԭ��Ӧ�Ĺ�����______________ ��(�I ���� II����III�� )
�ڻ�����( FeS2 ) ��SΪ- 1�ۣ���ɹ��� I �Ļ�ѧ��Ӧ����ʽ��______FeS2ʮ______=_____Fe2O3+ _____SO2��
�۹��� II �У�С��ͬѧ�� 500��C �� 10l kPa �����£���һ������ SO2 �� O2���뺬�д������ܱ������з�����Ӧ�����ŷ�Ӧ�Ľ��У������崫������������ֵ�Ũ�ȼ��±�
��Ӧʱ��/ s | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 100 |
c ( SO2 ) / ( mol L -1) | 10 | 7 | 5 | 3.5 | 2 | 1 | 1 | 1 | 1 |
c ( O2 ) / ( mol L -1) | 5 | 3.5 | a | 1.75 | 1 | 0.5 | b | 0.5 | 0.5 |
c ( SO3 ) / ( mol L -1) | 0 | 3 | 5 | 6.5 | 8 | 9 | 9 | 9 | 9 |
���ݷ��������� a ��b ��������ֵ�ֱ��ǣ�a =________��b = _______��С ��ͬѧ�ж� SO2 �� O2�� ��Ӧ 50 �����ƽ��״̬�����ǵ��ж�������___________��
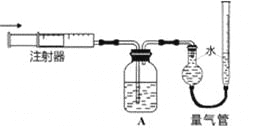
(3)Ũ������ľ̿�ڼ��������¿ɷ�����ѧ��Ӧ ��Ϊ�˼��鷴Ӧ���ijͬѧ���������ͼ��ʾ��ʵ��(���ּг�װ��ʡ��)���ش��������
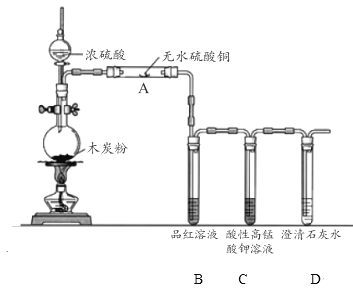
��Ũ������ľ̿��Ӧ�Ļ�ѧ����ʽ�� ________________��
��װ�� A �е�ʵ��������____________��֤���IJ�����___________ ��
��װ�� C ��������_______________ ��
�ܸ�ͬѧ��Ϊ�����ȥ��װ�� B Ҳ��ʵ�ֲ������֤����ͬѧ���ݵ�ʵ����������������__________________��