��Ŀ����
��֪���ڱ��У�Ԫ��Q��R��W��Y��Ԫ��X���ڡ�Y������ϼ��������ˮ������ǿ�ᡣ�ش��������⣺
��1��W��Q�����γ�һ�ָ��½ṹ�մɲ��ϡ�W���Ȼ�����ӳ���������ṹ��W��������ľ��������� ��
��2��Q�ľ�����ͬ���ϼ��ҿ����ת����������� ��
��3��R��Y�γɵĶ�Ԫ�������У�R��������ϼ۵Ļ�����Ļ�ѧʽ�� ��
��4����5��Ԫ�ص��⻯������У�������ṹ������ͬ���⻯��ķе�Ӹߵ������д����ǣ��ѧʽ�� ����ԭ���� ��
�ڵ���������ͬ���⻯��Ļ�ѧʽ������ṹ�ֱ��� ��
��5��W��Q���γɵĽṹ�մɲ��ϵ�һ�ֺϳɷ������£�W���Ȼ�����Q���⻯����ȷ�Ӧ�����ɻ�����W(QH2)4��HCL���壻W(QH2)4�ڸ����·ֽ�����Q���⻯����մɲ��ϡ�������ط�Ӧ�Ļ�ѧ����ʽ���������û�ѧʽ��ʾ���� ��
���𰸡���1��ԭ�Ӿ��塣��2��NO2��N2O4��3��As2S5����4����NH3> AsH3 > PH3,��Ϊǰ���к������,�����߹�����ͬ�����Ӽ���������ͬ���ڵ�������ͬ����SiH4��PH3��H2S�ṹ�ֱ�Ϊ�������壬������V�Ρ���5��SiCl4 + 4NH3 = Si(NH2)4 + 4HCl��3Si(NH2)4 = 8NH3 + Si3N4
������������ɽ����������W���Ȼ���Ϊ�������ͣ���ӦΪSiCl4��CCl4����W��Q�γɸ����մɣ��ʿ��ƶ�WΪSi����1��SiO2Ϊԭ�Ӿ��塣��2�������մɿ����뵽Si3N4,QΪN������NO2��N2O4֮����ת����ϵ����3��Y����������ĵ�ˮ����Ϊǿ�ᣬ����Si��N�����ڣ���ֻ����S��RΪAs������R����ۻ�����ӦΪAs2S5����4����ȻxΪPԪ�ء����⻯��е�˳��ΪNH3> AsH3 > PH3,��Ϊǰ���к�����������߹�����ͬ�����Ӽ���������ͬ����SiH4��PH3��H2S�ĵ�������Ϊ18�����ṹ�ֱ�Ϊ�������壬������V�Ρ���5���������������ĺ���ĸ�Ļ�ѧʽ����д����������ʣ�Ȼ����ƽ���ɡ�

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�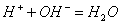 ��ʾ�Ļ�ѧ��Ӧ�� ( )
��ʾ�Ļ�ѧ��Ӧ�� ( ) 2AB(g)�ﵽƽ��ı�־��
2AB(g)�ﵽƽ��ı�־��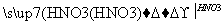 Mg(NO3)2
Mg(NO3)2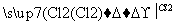 MgCl2
MgCl2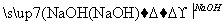 Mg(OH)2
Mg(OH)2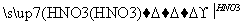 Mg(NO3)2
Mg(NO3)2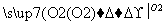 MgO
MgO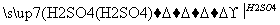 MgSO4
MgSO4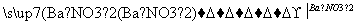 Mg(NO3)2
Mg(NO3)2