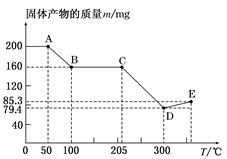
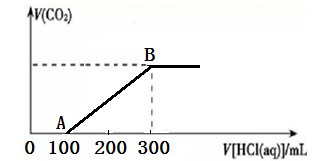
��Ŀ����
����������Һ����˫��ˮ,ҽ������������ɱ��������������ϴ�˿ڡ��ش������й�˫��ˮ������:
(1)������Ӧ��,H2O2�����������Եķ�Ӧ����������(�����)��
A��Na2O2+2HCl 2NaCl+H2O2 2NaCl+H2O2 |
B��Ag2O+H2O2 2Ag+O2��+H2O 2Ag+O2��+H2O |
C��2H2O2 2H2O+O2�� 2H2O+O2�� |
D��3H2O2+Cr2(SO4)3+10KOH 2K2CrO4+3K2SO4+8H2O 2K2CrO4+3K2SO4+8H2O |

(3)H2O2��ʱ����Ϊ��ҵ��Һ������,�С���ɫ��������������,�������ɿ�ҵ��Һ�е��軯��(��KCN),�����·�Ӧʵ��:KCN+H2O2+H2O
 A+NH3��,��ָ��������A�Ļ�ѧʽΪ������������������
A+NH3��,��ָ��������A�Ļ�ѧʽΪ������������������
(1)D��(2)B��(3)KHCO3
����

��ϰ��ϵ�д�
�����Ŀ

 ��4�������������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
��4�������������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�