��Ŀ����
��1��ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ�� ��
��2����4.48L����״��������ͨ��ˮ�еõ�0.05L��Һ��������Һ�����ʵ���Ũ���� ��
��3������100mL AlCl3��MgSO4�Ļ����Һ���ֳ����ȷݡ�
��������һ���м���10mL 4mol/L�İ�ˮ��ǡ����ȫ��Ӧ������AlCl3�백ˮ��Ӧ�����ӷ���ʽ�� ����������l mol/L NaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ�� �����ٵij��������ʵ����� ��
������һ���м���a mL 1mol/LBaCl2��Һ��ʹSO42��������ȫ��a= ��
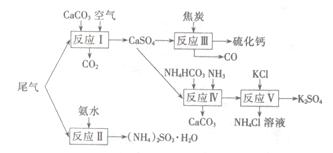
��1��Ca��OH��2��2NH4Cl CaCl2��2H2O��2NH3��
CaCl2��2H2O��2NH3��
��2��4 mol/L ��1�֣�
��3����A13+��3NH3��H2O=Al��OH��3����3NH4+
Al��OH��3��OH��=AlO2����2H2O
0.01 mol
�� 5
���� �����������ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ��Ca��OH��2��2NH4Cl
�����������ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ��Ca��OH��2��2NH4Cl CaCl2��2H2O��2NH3��
CaCl2��2H2O��2NH3��
�Ƹ���c=n/V,�ð��������ʵ�����4.48L/22.4L/mol=0.2mol������������Һ���ʵ���Ũ��Ϊ��0.2mol/0.05L="4" mol/L
�Ǣ�AlCl3�백ˮ��Ӧ�����ӷ���ʽ��A13+��3NH3��H2O=Al��OH��3����3NH4+���������ټ�����������������������Һ�����ӷ���ʽΪAl��OH��3��OH��=AlO2����2H2O���������ٵ����ʵ�����Ϊ�������Ƶ����ʵ���0.01mol
���ɢ�֪Al(OH)3�����ʵ���Ϊ0.01mol������NH3��H2O0.03mol������Mg2+����NH3��H2O��0.04mol-0.03mol��=0.01mol,��n��Mg2+��=0.005mol=n(SO42-����������Ҫ1mol/LBaCl2��Һ5mlʹSO42-������ȫ��
���㣺���ӷ�Ӧ����ؼ���

ijУ��ѧС��ѧ�����С�������Է��������IJⶨ����ʵ�顣�������£����������ݻ�����ȵ���ƿ�ռ����壬�����ռ����������ƿ���������ݼ��±�(�ѻ���ɱ�״���µ���ֵ)��
| ���� | ��ƿ�������������(g) |
| A | 48.4082 |
| B | 48.4082 |
| C | 48.4082 |
| D | 48.4342 |
| E | 48.8762 |
��֪��״���£���ƿ���ݻ�Ϊ0.293 L����ƿ�Ϳ�����������Ϊ48.4212 g��������ƽ����Է�������Ϊ29��A��B��C��D��E����ѧ���������塣
(1)�������������У��ܹ�ʹƷ����Һ��ɫ����(д��ѧʽ)________��
(2)D����Է���������________��
(3)�ڱ�״���£�11.2 L D�����к��й��õ��ӶԵ���ĿΪ________��
(4)A��B��C���ܵĻ�ѧʽ��________��
����������Һ����˫��ˮ,ҽ������������ɱ��������������ϴ�˿ڡ��ش������й�˫��ˮ������:
(1)������Ӧ��,H2O2�����������Եķ�Ӧ����������(�����)��
A��Na2O2+2HCl 2NaCl+H2O2 2NaCl+H2O2 |
B��Ag2O+H2O2 2Ag+O2��+H2O 2Ag+O2��+H2O |
C��2H2O2 2H2O+O2�� 2H2O+O2�� |
D��3H2O2+Cr2(SO4)3+10KOH 2K2CrO4+3K2SO4+8H2O 2K2CrO4+3K2SO4+8H2O |

(3)H2O2��ʱ����Ϊ��ҵ��Һ������,�С���ɫ��������������,�������ɿ�ҵ��Һ�е��軯��(��KCN),�����·�Ӧʵ��:KCN+H2O2+H2O
 A+NH3��,��ָ��������A�Ļ�ѧʽΪ������������������
A+NH3��,��ָ��������A�Ļ�ѧʽΪ������������������