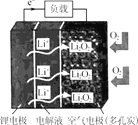
��Ŀ����
����Ŀ������������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�


ʵ�鲽�裺 ��A�м���4.4 g���촼(![]() )��6.0 g���ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143 ����֣�������������3.9 g���ش��������⣺
)��6.0 g���ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143 ����֣�������������3.9 g���ش��������⣺
(1)����B��������_________________��
(2)�������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������________ (����ȷ�𰸱��)��
A���������� B.��ȴ�� C.���貹�� D.��������
(3)��ϴ�Ӳ����У���һ��ˮϴ����ҪĿ����________���ڶ���ˮϴ����ҪĿ����___________��
(4)��Һ©����ʹ��ǰ����ϴ�ɾ���_______����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��_________________(����)��
a��ֱ�ӽ������������ӷ�Һ©���Ͽڵ���
b��ֱ�ӽ������������ӷ�Һ©���¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڷų�
(5)��ʵ���м�����������Ŀ����_________________��
(6)ʵ���м���������ˮMgSO4��Ŀ����_____________��
(7)����������У�����ѡ��װ����ȷ����_____________ (����) ��

(8)��ʵ��IJ�����_____________
a. 30% b. 40% c. 50% d. 60%
(9)�ڽ����������ʱ������130 �濪ʼ�ռ���֣���ʹʵ��IJ���ƫ_____________ (����������������)����ԭ����_____________��
���𰸡����������� B ϴ��������ʹ��� ϴ��̼������ ��© d ��ߴ���ת���� ���� b d �� ���ռ�����δ��Ӧ�����촼
��������
��1����װ��������B�Ĺ����֪������B������Ϊ���������ܣ�
��2���ڽ�����������У�����һ��ʱ��������Ƭ���ӣ�Ӧ�ò�ȡֹͣ���ȣ�����Һ��ȴ�������������Ƭ��
��ѡB��
��3����Ӧ�����ҺҪ�������ϴ�ӣ���ϴ�Ӳ����У���һ��ϴ�ӵ���ҪĿ���dz�ȥ�ִ�������ʹ���ñ���̼��������Һ�ȿ��Գ�ȥδϴ���Ĵ��ᣬҲ���Խ��������ܽ�ȣ����Եڶ���ˮϴ����ҪĿ���dz�ȥ��Ʒ�в�����̼�����ƣ�
��4����Һ©����ʹ��ǰ����ϴ�ɾ�����©�����������ܶȱ�ˮС�����������ܣ����ˮ���²㣬�����ϲ㣻��Һʱ��Ҫ�Ƚ�ˮ��ӷ�Һ©�����¿ڷų�����������Һ�����ʱ�رշ�Һ©���Ļ������ٽ��������������Ͽڷų���������ȷ��Ϊd���ʴ�Ϊ��d��
��5��������Ӧ�ǿ��淴Ӧ������Ӧ���Ũ�ȿ���ʹƽ�������ƶ�������һ�ַ�Ӧ���Ũ�ȣ�����ʹ��һ�ַ�Ӧ���ת������ߣ���˱�ʵ���н�����������Ŀ������ߴ���ת���ʣ�
��6��ʵ���м���������ˮ����þ��Ŀ������������������ˮ�֣�������и��
��7������������У��¶ȼƵ�ˮ����Ҫ����������ƿ��֧�ܿڴ�������ad����c��ʹ�õ�����������������ʹ��Ʒ����������ȫ���ռ�����ƿ�У����������װ�ð�װ��ȷ����b���ʴ�Ϊ��b��
��8����������ʵ���Ϊ��n=![]() =0.1mol�����촼�����ʵ���Ϊ��n=
=0.1mol�����촼�����ʵ���Ϊ��n=![]() =0.05mol��������������촼�ǰ���1��1���з�Ӧ���������������������������������Ҫ�������촼�����ʵ������㣬������������0.05mol������������ʵ�������ɵ����������������ʵ���Ϊ��
=0.05mol��������������촼�ǰ���1��1���з�Ӧ���������������������������������Ҫ�������촼�����ʵ������㣬������������0.05mol������������ʵ�������ɵ����������������ʵ���Ϊ��![]() ��0.03mol������ʵ���������������IJ���Ϊ��
��0.03mol������ʵ���������������IJ���Ϊ��![]() ��100%=60%���ʴ�Ϊd��
��100%=60%���ʴ�Ϊd��
��9���ڽ����������ʱ������130��㿪ʼ�ռ���ִ�ʱ�������к������촼�����ռ�������δ��Ӧ�����촼����˻ᵼ�²���ƫ�ߡ�

����Ŀ���ڱ�״���½��мס��ҡ�������ʵ�飺��ȡ200mLͬŨ�ȵ����ᣬ���벻ͬ������ͬһ��þ���Ͻ��ĩ���������壬�й����ݼ�¼���£�
ʵ����� | �� | �� | �� |
�Ͻ�����/g | 2.55 | 3.85 | 4.59 |
�����������/L | 2.80 | 3.36 | 3.36 |
�Իش�
(1)��������ʵ���Ũ��Ϊ________����������λ��Ч���֣�
(2)�Ͻ���þ�������ʵ���֮��_______��