��Ŀ����
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ���ⶨ�����Ũ����ʵ�����н���ʵ�顣�������գ�
��1������100 mL 0.10 mol/L NaOH����Һ��
����Ҫ�������裺������������ܽ��(��ȴ��)ת�ơ�ϴ��(����ϴ��Һ��������ƿ)�� �������ƺõ���Һ�����Լ�ƿ�У����ϱ�ǩ��
�ڳ��� g�������ƹ������������У���ƽ(�����롢����)�� �� ��
��2��ȡ20.00 mL����������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�ҺNaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������£�
�ٵζ��ﵽ�յ�ı�־�� ��
�ڸ����������ݣ��ɼ�����������Ũ��Լ (������λ��Ч����)��
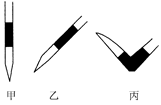
����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е� ��ѡ��ס��ҡ���֮һ����Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
�� ������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ��У� ��
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D���ζ�������,��������Һ������ƿ�⡣
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
��1������100 mL 0.10 mol/L NaOH����Һ��
����Ҫ�������裺������������ܽ��(��ȴ��)ת�ơ�ϴ��(����ϴ��Һ��������ƿ)�� �������ƺõ���Һ�����Լ�ƿ�У����ϱ�ǩ��
�ڳ��� g�������ƹ������������У���ƽ(�����롢����)�� �� ��
��2��ȡ20.00 mL����������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�ҺNaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������£�
| ʵ���� | NaOH��Һ��Ũ��(mol/L) | �ζ����ʱ�� NaOH��Һ��������(mL) | ����������Һ�����(mL) |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�ٵζ��ﵽ�յ�ı�־�� ��
�ڸ����������ݣ��ɼ�����������Ũ��Լ (������λ��Ч����)��
����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е� ��ѡ��ס��ҡ���֮һ����Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

�� ������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ��У� ��
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D���ζ�������,��������Һ������ƿ�⡣
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
��1���ٶ��� ��0.4 �ձ�(�������ҩ��
��2�������һ��NaOH��Һ���룬��Һ����ɫǡ�ñ�ɷۺ�ɫ ��0.11 mol/L �۱� �� DE
��2�������һ��NaOH��Һ���룬��Һ����ɫǡ�ñ�ɷۺ�ɫ ��0.11 mol/L �۱� �� DE
��������������������1��������һ�����һ�����ʵ���Ũ�ȵ���Һ�IJ��������Ǽ�����������ܽ��(��ȴ��)ת�ơ�ϴ��(����ϴ��Һ��������ƿ)�����ݡ� ҡ�ȡ� װƿ ����ǩ����n(NaOH)=" 0.100" L ��0.10 mol/L=0.01mol.m(NaOH)=0.01mol��40g/mol=0.4g.���Ҫ����0.4gNaOH. ���������У���ƽ(�����롢����)\�ձ���ҩ�ס���2��������������֪Ũ�ȵļ�ζ�δ֪Ũ�ȵ��ᣬ����ָʾ��������Һ�У����ζ��ﵽ�յ�ʱ�ῴ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ����ȷ���ζ��ﵽ���յ㡣��V(NaOH)= (22.62��22.72��22.80)ml��3=22.71ml.��ΪHClǡ�÷�Ӧʱ���ʵ����ı�Ϊ1:1.����c(HCl)=" (" 0.10 mol/L��22.71ml)��20.00ml=" 0.11" mol/L. �� ��ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����еı�������Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ����A���ζ��յ����ʱ���Ӷ���������ƫС������Һ�������ƫС�������������Һ��Ũ�Ⱦ�ƫ�͡�����B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ�������������Һ�����ʵ�����ƫ�٣��ζ�ʱ���ĵı���Һ�����ƫС�������������Һ��Ũ�Ⱦ�ƫ�͡�����C����ƿˮϴ��δ�������Ӱ��ʵ��ⶨ���������D���ζ�������,��������Һ������ƿ�⡣Ϊ�˽�������Һ���еζ�����Ҫ��μӱ���Һ������Һ���ƫ���ɴ˼����������Һ��Ũ�Ⱦ�ƫ�ߡ���ȷ��E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ����ʼ����ƫС����������ƫС�������ĵı���Һ�������ƫ�࣬�ɴ˼����������Һ��Ũ�Ⱦ�ƫ�ߡ���ȷ��

��ϰ��ϵ�д�
�����Ŀ