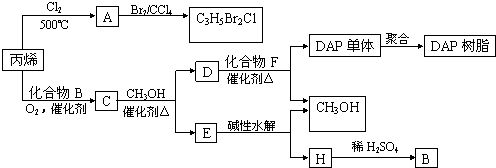
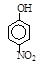��Ŀ����
 ��������Ԫ�أ��ֱ�λ��Ԫ�����ڱ���ǰ�ĸ���ͬ���ڣ���ԭ�������ܺ�Ϊ48�������ǿ���ɼס��ҡ����������ֵ��ʺ�A��B��C��D���ֻ�������мס���Ϊ�ǽ������ʣ�������Ϊ�������ʣ���Щ���ʼ��ת����ϵ��ͼ��ʾ����Ӧ������ʡ�ԣ�
��������Ԫ�أ��ֱ�λ��Ԫ�����ڱ���ǰ�ĸ���ͬ���ڣ���ԭ�������ܺ�Ϊ48�������ǿ���ɼס��ҡ����������ֵ��ʺ�A��B��C��D���ֻ�������мס���Ϊ�ǽ������ʣ�������Ϊ�������ʣ���Щ���ʼ��ת����ϵ��ͼ��ʾ����Ӧ������ʡ�ԣ���ش��������⣺
��1����ɶ���Ԫ�������ڱ��е�λ��
��2���A+��
| �� |
| ||
| ||
��3���ڼ��������£�������̬D�ɷ�����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ
| ||
| ||
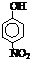
��4����21.6g A���������ҷ�Ӧ����B��A��B������ͼʾת��Ϊ��ҺI����ҺII�����������Ӧ��ǡ�ý�����ȫ��������ҺI��ͨ��
BΪ��ɫ���壬BΪFe3O4���ס���Ϊ�ǽ������ʣ��ʼס�����һ��ΪH2��һ��ΪO2��ԭ�������ܺ�Ϊ48��������Ԫ�ص�ԭ������Ϊ48-1-8-26=13��ΪAlԪ�أ�
������Ϊ�������ʣ���A+�ס���+D��Fe3O4+�ס���+D��֪����ΪFe���ʱ�ΪAl��A��Fe3O4������ͬ��Ԫ�أ���ΪH2��DΪH2O������ΪO2�����A
| �� |
FeO
| ���� |
| ���� |
BΪ��ɫ���壬BΪFe3O4���ס���Ϊ�ǽ������ʣ��ʼס�����һ��ΪH2��һ��ΪO2��ԭ�������ܺ�Ϊ48��������Ԫ�ص�ԭ������Ϊ48-1-8-26=13��ΪAlԪ�أ�
������Ϊ�������ʣ���A+�ס���+D��Fe3O4+�ס���+D��֪����ΪFe���ʱ�ΪAl��A��Fe3O4������ͬ��Ԫ�أ���ΪH2��DΪH2O������ΪO2�����A
| �� |
FeO
| ���� |
| ���� |
��1����ΪFe��FeԪ����26��Ԫ�أ��������ڱ��е������ڵڢ��壻
BΪFe3O4������Ϊ������������
CΪAl2O3���������ͻ���ϻ���������ԭ�ϣ�
�ʴ�Ϊ���������ڵڢ��壻���������������ͻ���ϻ���������ԭ�ϣ�
��2��A+��
| �� |
| ||
�ʴ�Ϊ��6FeO+O2
| ||
��3���ڼ��������£�Fe��ˮ������Ӧ������������������������Ӧ����ʽΪ��3Fe+4H2O��g��
| ||
�ʴ�Ϊ��3Fe+4H2O��g��
| ||
��4����21.6gFeO�����ʵ���Ϊ
| 21.6g |
| 72g/mol |
| 1 |
| 3 |
| 2 |
| 3 |
�ʴ�Ϊ��0.1mol��

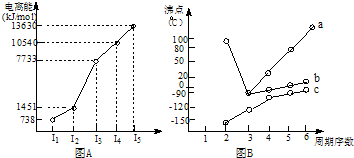
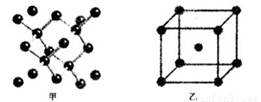
��18�֣�(1)I��������ij����Ԫ��M�ĵ������������ͼ(A)��ʾ����MԪ��λ�����ڱ��ĵ� �塣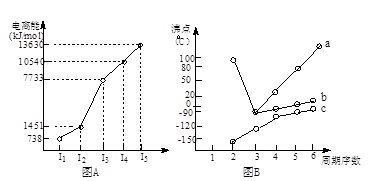
II��ͼB����c���Ա������ ��Ԫ���⻯��ķе�ı仯���ɡ���λͬѧ��ij����Ԫ���⻯��ķе�ı仯���ƻ�������������a��b������Ϊ��ȷ���ǣ�__________(�a����b��)
III�������л�����۷е���±���
| �� | CH4 | CH3CH3 | CH3(CH2)2CH3 | �������� | 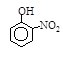 | 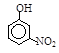 |  |
| �е�/�� | ��164 | ��88.6 | ��0.5 | �۵�/�� | 45 | 96 | 114 |
��
��
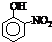
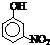
(2)COCl2�׳ƹ�����������Cԭ�Ӳ�ȡ �ӻ��ɼ�������̼��ԭ��֮�乲�ۼ�����
________(����ĸ)��
a��2���Ҽ���b��2���м���c��1���Ҽ���1���м���
(3)��������(22Ti) ���Ǽ�ͭ��������֮������㷺ʹ�õĵ����ֽ������Իش�
I��TiԪ�صĻ�̬ԭ�ӵļ۵��Ӳ��Ų�ʽΪ ��
II����֪Ti3�����γ���λ��Ϊ6������������ɫ����ɫ���ֺ��Ѿ��壬����ɾ�ΪTiCl3��6H2O��Ϊ�ⶨ�����־���Ļ�ѧʽ�����������ʵ�飺a���ֱ�ȡ�����������־������Ʒ�����Һ��b����������Һ�зֱ����AgNO3��Һ����������ɫ������c��������ȫ��ֱ���˵����ݳ�������ϴ�Ӹ����������ֲ����ij���������ϵΪ����ɫ����Ϊ��ɫ�����2/3������ɫ���������Ļ�ѧʽΪ ��
��18�֣�(1)I��������ij����Ԫ��M�ĵ������������ͼ(A)��ʾ����MԪ��λ�����ڱ��ĵ� �塣
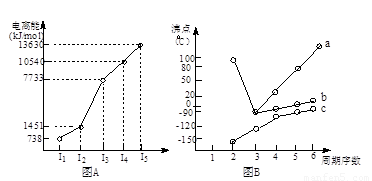
II��ͼB����c���Ա������ ��Ԫ���⻯��ķе�ı仯���ɡ���λͬѧ��ij����Ԫ���⻯��ķе�ı仯���ƻ�������������a��b������Ϊ��ȷ���ǣ�__________(�a����b��)
III�������л�����۷е���±���
|
�� |
CH4 |
CH3CH3 |
CH3(CH2)2CH3 |
�������� |
|
|
|
|
�е�/�� |
��164 |
��88.6 |
��0.5 |
�۵�/�� |
45 |
96 |
114 |
�������ʽṹ���ۣ�����Щ�������ܵó�����ؽ�����(����д2��)��
��
��
(2)COCl2�׳ƹ�����������Cԭ�Ӳ�ȡ �ӻ��ɼ�������̼��ԭ��֮�乲�ۼ�����
________(����ĸ)��
a��2���Ҽ���b��2���м���c��1���Ҽ���1���м���
(3)��������(22Ti) ���Ǽ�ͭ��������֮������㷺ʹ�õĵ����ֽ������Իش�
I��TiԪ�صĻ�̬ԭ�ӵļ۵��Ӳ��Ų�ʽΪ ��
II����֪Ti3�����γ���λ��Ϊ6������������ɫ����ɫ���ֺ��Ѿ��壬����ɾ�ΪTiCl3��6H2O��Ϊ�ⶨ�����־���Ļ�ѧʽ�����������ʵ�飺a���ֱ�ȡ�����������־������Ʒ�����Һ��b����������Һ�зֱ����AgNO3��Һ����������ɫ������c��������ȫ��ֱ���˵����ݳ�������ϴ�Ӹ����������ֲ����ij���������ϵΪ����ɫ����Ϊ��ɫ�����2/3������ɫ���������Ļ�ѧʽΪ ��