��Ŀ����
��10�֣�ij��ɫ��Һ���ܺ������������еļ���:
| A���Ȼ��� | B������ | C���������� | D����������� (E)������ (F)̼����.�����Һ�м�������ϡ����,��dz��ɫ�ij�������,ͬʱ���������.�������г�������ζ,��ʹ�����ʯ��ˮ�����,����ʹƷ����Һ��ɫ������֪����������ƵĻ�ѧʽΪNa2S2O3, �����������ϡ����ķ�ӦʽΪ�� |
��������ʵ������ش���������
(1)����ʹƷ����Һ��ɫ,˵���������в���____________(�����ʽ).[��Դ:Z|xx|k.Com]
(2)����ɫ��Һ�����ٿ��ܴ����ļ�������?��д��ȫ�����ܵ����(��д��Ӧ����ĸ).
��һ�������______ ______,�ڶ��������____ ________,�����������_________ ___,�����������______ ______.
(�ɲ�����,Ҳ�ɲ���)
��10�֣���ÿ��2�֣�
��1��SO2
��2��B C F BDF BCDF �����������������֣�
����

 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д���1����Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ��������������֮������֪��Ԫ�ؾ����������ʣ�
 ��
��




 ��
��
�Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��_______________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������__________���ѧʽ����
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü��ѡ��________��
��2��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
|
������ |
|
|
������ |
|
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ�����n��������Լ�Y�����V���Ĺ�ϵ��ͼ��ʾ��

����Y�����ᣬ����Һ�к��еĽ�����������_________��ab�η�����Ӧ�����ӷ���ʽΪ_______________��ͼ��oa�βμӷ�Ӧ�������ӵ����ʵ���֮��Ϊ___________________��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ____________________��
�����������ӵ�ˮ�⣬����H����OH����Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ_____________________________________________������������ǰ���������ں���ǰ���ͼ��ں��˳�����У���
�� 14 �֣�ij��ɫ����Һ���ܺ����������ӣ�K+��Fe3+��Cu2+��Ba2+��AlO��NO3����CO32����SO32����SO42����Br����I����Cl����ȡ����Һ�����²������ʵ�顣
��1����������±������е�ʵ�������ش�������й����⣺
|
��ʵ���� |
��ش����� |
|
��ȡԭ��Һ�������μ�������ˮ���������������ˣ���Һ��CCl4����CCl4��δ��ɫ�� |
�ٸò�ʵ��˵����Һ�в����� ����_________________�� |
|
��ȡԭ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬������������������Ϊ����ɫ�� |
�ڸò�ʵ�鷢����Ӧ�����ӷ���ʽΪ _________________�� |
|
��ȡԭ��Һ�������������ữ���백ˮ�а�ɫ�������ɣ��������������ˮ����������ʧ�� |
�۲�����ɫ���������ӷ���ʽΪ _________________�� |
|
��ȡԭ��Һ�������μ�2�����Ը��������Һ����ɫ������ȥ�� |
�ܸò�ʵ��˵����Һ�д��� ���� _________________�� |
|
��ȡԭ��Һ������������Ba��NO3��2��Һ������ɫ���������ˣ���ϴ�Ӻ�ij����м����������ᣬ���������ܽ⣬����ɫ��������� |
��δ�ܽ�İ�ɫ������ ��������ͨ��Ʒ����Һ�������� �� |
|
��ȡ�����������Һ����HNO3�ữ���ټ�AgNO3��Һ����Һ��������ɫ������ |
�ò����Ƿ������� ����С����ޡ����� |
��2�����ϣ�����Һ��pH ���������������������7������Һ��һ�����ڵ��������� ������ȷ���Ƿ���ڵ������� ������ȡԭ��Һ�������Ƿ���ڣ��Ƿ�Ҫʹ���Լ�������Ҫʹ���Լ�����ʵ�鷽���� ����Ҫʹ���Լ���ѡ�õ��Լ��ǣ���ʹ�õ��Ⱥ�˳������д����
��
 ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�
ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�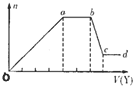 �� I��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�
�� I��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�

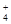 ��Na+��Cl����MnO
��Na+��Cl����MnO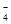
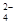 ��CO
��CO