��Ŀ����
��1����Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ��������������֮������֪��Ԫ�ؾ����������ʣ�
 ��
��




 ��
��
�Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��_______________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������__________���ѧʽ����
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü��ѡ��________��
��2��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
|
������ |
|
|
������ |
|
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ�����n��������Լ�Y�����V���Ĺ�ϵ��ͼ��ʾ��

����Y�����ᣬ����Һ�к��еĽ�����������_________��ab�η�����Ӧ�����ӷ���ʽΪ_______________��ͼ��oa�βμӷ�Ӧ�������ӵ����ʵ���֮��Ϊ___________________��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ____________________��
�����������ӵ�ˮ�⣬����H����OH����Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ_____________________________________________������������ǰ���������ں���ǰ���ͼ��ں��˳�����У���
��1����Sn+2HCl =SnCl2+H2����1�֣��� SnCl2+Cl2=SnCl4��1�֣�
��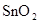 ��1�֣� ��
��1�֣� ��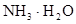 ��1�֣�
��1�֣�
��3����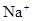 ��1�֣�CO32-+2
H+=CO2��+H2O
��1�֣�CO32-+2
H+=CO2��+H2O  11��2
11��2
��Al(OH)3 +OH-- =AlO2-+2 H2O
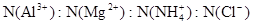 ��2��1��4��12
��2��1��4��12
��������
�����������1����Ϊ�ڢ�A��Ԫ�أ����ϼ���+4��+2���йط�Ӧ������������Ӧ�仯�������������ᷴӦ��Sn+2HCl =SnCl2 + H2��������Ӧ�����Һ��ͨ��������SnCl2 + Cl2 =SnCl4����SnCl4��Һ���������գ�������FeCl3��Һ��Ӧ�ı仯��ˮ��ƽ�������ƶ���HCl�ӷ���ʣ��Fe(OH)3�����ȶ����ȷֽ�����Fe2O3����ȿ�֪SnCl4��Һ���������գ��ɵ�SnO2;Sn(OH)2���ܰ���ʽ���룬���ܰ���ʽ���룬����������������Կ�������ǿ��ǿ����ѡ�������Ʊ����Ӱ�ˮ����2����ɫϡ��Һ��˵��û��Cu2+���������ᣬ�ܺ�AlO2-��SiO32-���ɳ�����ab�γ����������ӣ���CO32-���뷴Ӧ��bc�γ������٣���Al(OH)3�ܽ⣬���������������Ӵ��ڣ����ܴ��ڵĽ���������ֻ��Na+��ab�η�����Ӧ�����ӷ���ʽΪCO32- + 2 H+ = CO2��+H2O��bc����������һ����һ��Ϊ����3Ħ��������ƶϳ�Al(OH)3+3HCl=AlCl3+3H2O���������غ㣬��֪AlO2-��1Ħ����oa�����������ĸ��൱������12Ħ����AlO2-��������1Ħ����ʣ���11Ħ����SiO32-���ģ������֪SiO32-Ϊ11/2Ħ��������SiO32-��AlO2-�����ʵ���֮��Ϊ11:2������Y��NaOH��Һ���ܺ�Mg2+��Al3+��Ӧ���ɳ�����ab�γ����������ӣ���NH4+���뷴Ӧ��bc�γ������٣���Al(OH)3�ܽ⣬bc�η�Ӧ�����ӷ���ʽΪAl(OH)3 + OH-- = AlO2- + 2 H2O�����������������Ӵ��ڣ����ܴ��ڵ�������ֻ��Cl-��bc������NaOHһ����һ��ΪNaOH1Ħ����������ƶ�Al3+1Ħ����oa������NaOH�ĸ��൱��NaOH4Ħ����Al3+����NaOH3Ħ�������֪Mg2+0.5Ħ����ab�ζ���֪NH4+2Ħ�������ݵ���غ㣬����֪Cl-6Ħ����������ǵ����Ӹ�����2��1��4��12
���㣺����������仯��������ʡ����ӵĹ��桢ͼ��ͼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
