��Ŀ����
ij�����A������KAl��SO4��2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
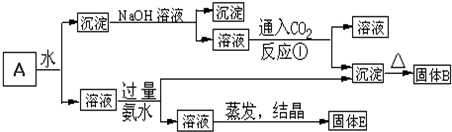
�ݴ��жϣ�
��1������B�������ʵĻ�ѧʽΪ ��
��2������E�������ʵĻ�ѧʽΪ ��
��3����Ӧ����ͨ������CO2�����ӷ���ʽΪ ��
��4����������ͼ�м��������ˮ�����ӷ���ʽΪ ��
��5����Ҫ������1mol KAl��SO4��2����Һ��SO42+ǡ����ȫ����������Ҫ���� mol Ba��OH��2�����ӷ���ʽΪ ��

�ݴ��жϣ�
��1������B�������ʵĻ�ѧʽΪ
��2������E�������ʵĻ�ѧʽΪ
��3����Ӧ����ͨ������CO2�����ӷ���ʽΪ
��4����������ͼ�м��������ˮ�����ӷ���ʽΪ
��5����Ҫ������1mol KAl��SO4��2����Һ��SO42+ǡ����ȫ����������Ҫ����
���㣺���ʷ��롢�ᴿ��ʵ�鷽�����,þ��������Ҫ������
ר�⣺Ԫ�ؼ��仯����
������KAl��SO4��2����ˮ��Al2O3��Fe2O3��������ˮ�������A��ˮ�ܽ����Һ����KAl��SO4��2��������Al2O3��Fe2O3��
������м�NaOH��Һ��Fe2O3����Ӧ��Al2O3����NaOH��Һ��Ӧ����NaAlO2����NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������Al��OH��3���ȷֽ�����Al2O3��
����Һ�мӹ�����ˮ����Һ�������ˮ��Ӧ��Al3+���������õ�����������������Һ��ʣ�����K2SO4 �ͣ�NH4��2SO4�������������ᾧ���õ�����K2SO4 �ͣ�NH4��2SO4��
��NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������̼���ƣ�����̼�����ƣ�ȡ����CO2��������
������м�NaOH��Һ��Fe2O3����Ӧ��Al2O3����NaOH��Һ��Ӧ����NaAlO2����NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������Al��OH��3���ȷֽ�����Al2O3��
����Һ�мӹ�����ˮ����Һ�������ˮ��Ӧ��Al3+���������õ�����������������Һ��ʣ�����K2SO4 �ͣ�NH4��2SO4�������������ᾧ���õ�����K2SO4 �ͣ�NH4��2SO4��
��NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������̼���ƣ�����̼�����ƣ�ȡ����CO2��������
���
�⣺KAl��SO4��2����ˮ��Al2O3��Fe2O3��������ˮ�������̿�֪�������A��ˮ�ܽ����Һ����KAl��SO4��2��������Al2O3��Fe2O3��
������м�NaOH��Һ��Fe2O3����Ӧ��Al2O3����NaOH��Һ��Ӧ����NaAlO2����NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������Al��OH��3���ȷֽ����ɹ���BΪAl2O3��
����Һ�мӹ�����ˮ����Һ�������ˮ��Ӧ��Al3+���������õ�����������������Һ��ʣ�����K2SO4 �ͣ�NH4��2SO4�������������ᾧ���õ��Ĺ���EΪK2SO4 �ͣ�NH4��2SO4��
��1��������������֪��BΪAl2O3���ʴ�Ϊ��Al2O3��
��2��������������֪��E�к�K2SO4����NH4��2 SO4���ʴ�Ϊ��K2SO4����NH4��2 SO4��
��3����Ӧ����ͨ������CO2�����ӷ���ʽΪ2AlO2-+CO2+3H2O�TCO32-+2Al��OH��3�����ʴ�Ϊ��2AlO2-+CO2+3H2O�TCO32-+2Al��OH��3����
��4�����������ˮ�����ӷ���ʽΪAl3++3NH3?H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
��5������1mol KAl��SO4��2����Һ��SO42+ǡ����ȫ��������2molSO42-������Ҫ2molBa��OH��2�����������ӷ�ӦΪAl3++4OH-+2SO42-+2Ba2+=AlO2-+2BaSO4��+2H2O��
�ʴ�Ϊ��2��Al3++4OH-+2SO42-+2Ba2+=AlO2-+2BaSO4��+2H2O��
������м�NaOH��Һ��Fe2O3����Ӧ��Al2O3����NaOH��Һ��Ӧ����NaAlO2����NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������Al��OH��3���ȷֽ����ɹ���BΪAl2O3��
����Һ�мӹ�����ˮ����Һ�������ˮ��Ӧ��Al3+���������õ�����������������Һ��ʣ�����K2SO4 �ͣ�NH4��2SO4�������������ᾧ���õ��Ĺ���EΪK2SO4 �ͣ�NH4��2SO4��
��1��������������֪��BΪAl2O3���ʴ�Ϊ��Al2O3��
��2��������������֪��E�к�K2SO4����NH4��2 SO4���ʴ�Ϊ��K2SO4����NH4��2 SO4��
��3����Ӧ����ͨ������CO2�����ӷ���ʽΪ2AlO2-+CO2+3H2O�TCO32-+2Al��OH��3�����ʴ�Ϊ��2AlO2-+CO2+3H2O�TCO32-+2Al��OH��3����
��4�����������ˮ�����ӷ���ʽΪAl3++3NH3?H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
��5������1mol KAl��SO4��2����Һ��SO42+ǡ����ȫ��������2molSO42-������Ҫ2molBa��OH��2�����������ӷ�ӦΪAl3++4OH-+2SO42-+2Ba2+=AlO2-+2BaSO4��+2H2O��
�ʴ�Ϊ��2��Al3++4OH-+2SO42-+2Ba2+=AlO2-+2BaSO4��+2H2O��
���������⿼������ķ��롢�ᴿʵ�鷽������ƣ�Ϊ��Ƶ���㣬���������л��������ᴿ�ķ����������仯�������ʡ�Al2O3��������Ϊ���Ĺؼ���ע�����ʵ����ʼ������ķ�Ӧ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
�������ֱ����뷴Ӧ����ʽ��Ӧ����ȷ���ǣ�������
A���Ҵ���������Ũ�������ʱ���ȵķ�Ӧ��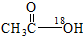 +CH3CH2OH +CH3CH2OH
 +H2O +H2O | |||
| B�����Լ���ȼ�ϵ�صĸ�����ӦʽΪ��CH4+10OH--8e-=CO32-+7H2O | |||
C��������ϡ����������ʱ��ˮ�ⷴӦ��C12H22O11�����ǣ�+H2O
| |||
D������������Ӧ�� +HNO3��Ũ�� +HNO3��Ũ��
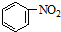 |
����˵������ȷ���ǣ�������
| A��ͭ�ڳ�ʪ�Ŀ����в��ᱻ��ʴ |
| B�������Ŀ�ʴ�Ժ�ǿ |
| C����ͨ����û��һ�����۵� |
| D�������£���������������������������� |
��֪C-C�������Ƽ���������ת�����ڽṹ��ʽΪͼ��ʾ����������˵������ȷ���ǣ�������
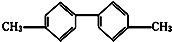

| A�������Ƿ�������Ҳ�DZ���ͬϵ�� |
| B��������������10��̼ԭ�Ӵ���ͬһƽ���� |
| C��������������8��̼ԭ�Ӵ���ͬһƽ���� |
| D�����������ϵ�һ��ȡ������������� |
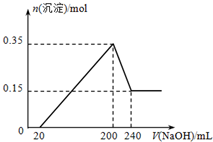 ��һ��������þ���������Ͷ��200mL�����У�����ȫ���ܽ����������Һ�м���5mol?L-1 NaOH��Һ�����ɳ��������ʵ���n�����NaOH��Һ�����V�ı仯��ͼ��ʾ����ͨ������ش�
��һ��������þ���������Ͷ��200mL�����У�����ȫ���ܽ����������Һ�м���5mol?L-1 NaOH��Һ�����ɳ��������ʵ���n�����NaOH��Һ�����V�ı仯��ͼ��ʾ����ͨ������ش�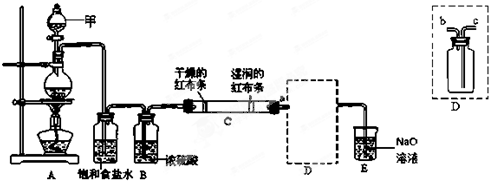
 ��֪��25�桢1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺
��֪��25�桢1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺