��Ŀ����
(��)����ѡ����
����˵������ȷ����________��
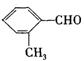
A����ϩ��������8���Ҽ���1���м�
B����SiO2�����У�1��Siԭ�Ӻ�2��Oԭ���γ�2�����ۼ�
C��NF3�ķе��NH3�ķе�͵ö࣬����ΪNH3���Ӽ��������NF3ֻ�з��»���
D��NCl3��BC13�����У�����ԭ�Ӷ����� sp3�ӻ�
E��SO3��CO32����Ϊ�ȵ����壬SO3�Ǽ��Է���
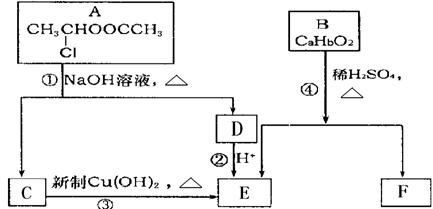
(��)������ʹ�ý�������ʷ�����У�������ͭ��������֮�����ֽ����㷺Ӧ�õĽ�������ѧ��Ԥ������(Ti)��������Ϊ��δ�����͵Ľ��������Իش��������⣺
(1)TiԪ����Ԫ�����ڱ��е�λ���ǵ�________���ڵ�________�壻���̬ԭ�ӵĵ����Ų�ʽΪ________��
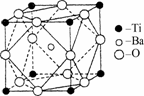
(2)��Ti�Ļ������У����Գ��֣�2����3����4���ֻ��ϼۣ������ԣ�4�۵�Ti��Ϊ�ȶ���ƫ���ᱵ�����ȶ��Ժã���糣���ߣ���С�ͱ�ѹ������Ͳ���������ж���Ӧ�ã�ƫ���ᱵ�����о����Ľṹʾ��ͼ��ͼ��ʾ�����Ļ�ѧʽ��________������Ti4+������λ��Ϊ________��Ba2+������λ��Ϊ________��
(3)�����µ�TiCl4���д̼��Գ�ζ����ɫ��Һ�壬�۵㣭23.2�棬�е�136.2�棬����TiCl4��________���壮
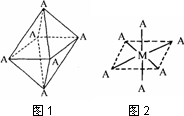
(4)��֪Ti3+���γ���λ��Ϊ6��������ռ乹��Ϊ�������壬����ͼ1��ʾ������ͨ����������ͼ2��ʾ�ķ�������ʾ��ռ乹��(����A��ʾ���壬M��ʾ����ԭ��)����λ������[Co(NH3)4Cl2]�Ŀռ乹��ҲΪ�������ͣ�����________��ͬ���칹�壮
(��)(1)��֪����������ӵĿռ�ṹ����ͼ��ʾ����������ԭ�Ӳ�ȡ________�ӻ���(2)R��1��36��Ԫ����δ�ɶԵ���������ԭ�ӣ�R3+����Һ�д�������ת����ϵ��

R3+![]() R(OH)3
R(OH)3![]() [R(OH)4]��
[R(OH)4]��
�ٻ�̬Rԭ�ӵļ۵����Ų�ʽΪ________��
��[R(OH)4]���д��ڵĻ�ѧ����________��
A�����Ӽ�
B�����Լ�
C���Ǽ��Լ�
D�����

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д�


 ����������ԭ�Ӳ����ܹ�ƽ��
����������ԭ�Ӳ����ܹ�ƽ��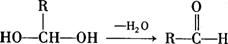
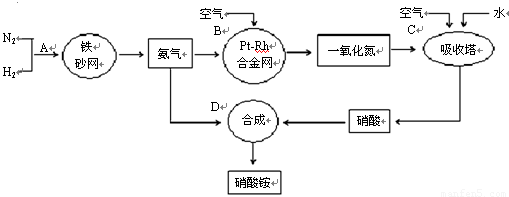
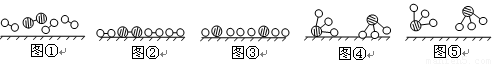
 �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���
��
��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���
��
�� 7N2+12H2O��NOҲ�����Ƶķ�Ӧ��
7N2+12H2O��NOҲ�����Ƶķ�Ӧ��