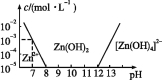
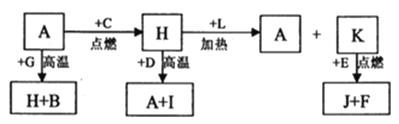
��Ŀ����
����Ŀ��ʵ�������ܶ�Ϊ1.25 g��mL��1����������Ϊ36.5%��Ũ��������240 mL 0.1 mol��L��1�����ᣬ��ش��������⣺
��1��Ũ��������ʵ���Ũ��Ϊ ��
��2������240 mL 0.1 mol��L��1������Ӧ��ȡŨ������� mL��Ӧѡ������ƿ�Ĺ��______mL��
��3������ʱ������ȷ�IJ���˳����������ĸ��ʾ��ÿ����ĸֻ����һ���� ��
A����30 mLˮϴ���ձ��ڱںͲ�����2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30 mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע������ƿ��
D��������ƿ�ǽ�����ҡ��
E������ ��ˮ��ʹ��Һ��Һ��ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶��� ��
��4������A�У���ϴ��Һ����������ƿ����Ŀ����
��5����ʵ������г������������δ�����
��������ˮʱ���������˿̶���
��������ƿ��ת����Һʱ��������Һ����
���𰸡�
��1��12.5 mol��L��1��
��2��2��250��
��3��BCAFED����ͷ�ι���1��2 cm
��4����֤����ȫ��ת������ƿ
��5������������������������
��������
�����������1��Ũ��������ʵ���Ũ��c=![]() =12.5mol/L���ʴ�Ϊ��12.5mol/L��
=12.5mol/L���ʴ�Ϊ��12.5mol/L��
��2��Ҫ����240ml����Һ��Ӧѡ��250ml������ƿ������ҪŨ��������ΪV��������Һϡ��ǰ���������ʵ����ʵ��������V��12.5mol/L=250mL��0.1molL-1�����V=2.0ml������ƿֻ��һ���̶��ߣ���ֻ�����ƺ��������Ӧ���������Һ�����������������ԭ��Ӧѡ��250mL����ƿ���ʴ�Ϊ��2.0��250��
��3��Ũ��Һ����ϡ��Һ��һ��������裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ������Ʋ���ΪBCAFED������E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶������У�F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2 cm�����ʴ�Ϊ��BCAFED����ͷ�ιܣ�1��2 cm��
��4������A�У���ϴ��Һ����������ƿ����Ŀ���DZ�֤����ȫ��ת������ƿ���ʴ�Ϊ����֤����ȫ��ת������ƿ��
��5��ʵ������������ֶ�ʵ����������IJ�������Ӧ������������Һ����������ˮʱ���������˿̶��ߣ�������Һ�����ƫ��Ũ��ƫС����Ҫ�������ƣ���������ƿ��ת����Һʱ��������Һ�������������ʵ����ʵ���ƫС��Ũ��ƫС����Ҫ�������ƣ��ʴ�Ϊ�����������ƣ����������ơ�

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�