��Ŀ����
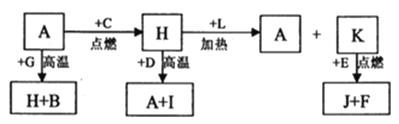
����Ŀ��A��B��C��D��E��F�dz������ʣ�����A���������Ӧ����㷺�Ľ�����Ԫ��D�ǵؿ��к������Ľ���Ԫ�أ�DԪ�غ�EԪ�������ڱ������ڡ�G��H��I��J��K��L�dz������������G�ڳ���������ɫҺ�壬H�Ǻ�ɫ���塣������������ת����ϵ����ͼ��ʾ��
��ش��������⣺
��1��Ԫ��A��Ԫ�����ڱ��е�λ��Ϊ ��
��2��д��K�ĵ���ʽ ��
��3��G��������ѧ��������Ϊ ��
��4��д��D������������Һ��Ӧ�����ӷ���ʽ ��
��5��д��A��G��Ӧ�Ļ�ѧ����ʽ ��
��6��д��H��D��Ӧ�Ļ�ѧ����ʽ ��
��7����֪F��ȼ����ΪakJ/mol,L��ȼ����ΪbkJ/mol,��д��F����L���Ȼ�ѧ����ʽ_______________________��
���𰸡���1���������� �ڢ���
��2��![]() ��3�����Լ��ۼ�
��3�����Լ��ۼ�
��4��2Al+2OH-+2H2O==2AlO2-+3H2��
��5��3Fe+4H2O(g)![]() Fe3O4+4H2
Fe3O4+4H2
��6��3Fe3O4+8Al![]() 4Al2O3+9Fe
4Al2O3+9Fe
��7��C��s��+1/2O2��g��=CO��g�� ��H=-��a-b��kJ/mol
��������
���������A��B��C��D��E��F�dz������ʣ�����A���������Ӧ����㷺�Ľ�������AΪ����Ԫ��D�ǵؿ��к������Ľ���Ԫ�أ���DΪ����G��H��I��J��K��L �dz������������ G �ڳ���������ɫҺ������GΪH2O��H �Ǻ�ɫ������ת����ϵ�ж�HΪFe3O4��˵��CΪO2��A��Fe��+G��H2O��=H��Fe3O4��+B��H2��������GΪH2O��HΪFe3O4��BΪH2��H��Fe3O4��+D��Al��=A��Fe��+I��Al2O3��������H+L=A+K��֪��LΪΪCO��KΪCO2��D Ԫ�غ� E Ԫ�������ڱ������ڣ����K+E=J+F�ƶ�EΪMg��JΪMgO��FΪC���ݴ�����
��1��Ԫ�� A ΪFe����Ԫ�����ڱ��е�λ��Ϊ���������ڣ�VIII����
��2��KΪCO2��������̼�ĵ���ʽΪ��![]() ��
��
��3��H��Fe3O4���� D ��Al����Ӧ�Ļ�ѧ����ʽ��3Fe3O4+8Al![]() 4Al2O3+9Fe��
4Al2O3+9Fe��
��4��D��������������Һ��Ӧ����д���÷�Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2A1O2-+3H2����
��5��A��Fe���� G��H2O�� ��Ӧ�Ļ�ѧ����ʽΪ��3Fe+4H2O��g��![]() Fe3O4+4H2��
Fe3O4+4H2��
��6��K��CO2�� �� E��Mg�� ��Ӧʱ����������Ϊ��þ������ȼ�գ�������ҫ�۵İ⣬��ƿ�ڻ�ƿ�׳��ְ�ɫ����ͺ�ɫ��������Ӧ�Ļ�ѧ����ʽΪ2Mg+CO2![]() 2MgO+C��
2MgO+C��
��7����֪C��ȼ����Ϊa kJ/mol��CO��ȼ����Ϊb kJ/mol����C��s��+O2��g��=CO2��g����H=-akJ/mol��CO��g��+1/2O2��g��=CO2��g����H=-bkJ/mol��C����CO���Ȼ�ѧ����ʽΪC��s��+1/2O2��g��=CO��g����H=��b-a��kJ/mol��

����Ŀ���������ʷ������ȷ����ǣ� ��
ѡ�� | �� | �� | ���������� | �� |
A | H2CO3 | ���� | CaO | ���� |
B | H2SO4 | �ռ� | Na2O | С�մ� |
C | HNO3 | ��ˮ | Al2O3 | ʯ��ʯ |
D | NaHCO3 | ��ʯ�� | Al��OH��3 | ʳ�� |
A.A
B.B
C.C
D.D