题目内容
把12.6g的镁铝合金的粉末放入过量盐酸中,得到13.44L气体(标况下)。试计算:
(1)该合金中铝的质量分数
(2)该合金中镁和铝的物质的量之比
(1)该合金中铝的质量分数
(2)该合金中镁和铝的物质的量之比
(1)W(Al) = 42.9%
(2)n(Mg): n(Al)="0." 3: 0. 2=3:2
(2)n(Mg): n(Al)="0." 3: 0. 2=3:2
设合金中镁和铝的物质的量分别为X mol,Y mol,
n(H2)=" 13.44/22.4" =" 0.6" mol
Mg+2H+===Mg2++H2↑
1 mol 1 mol
X mol Xmol
2Al+6H+===2Al3++3H2↑
2 mol 3 mol
Y mol 1.5 Y mol

(1)W(Al)= = 42.9%
= 42.9%
(2)n(Mg): n(Al)="0." 3: 0. 2=3:2
n(H2)=" 13.44/22.4" =" 0.6" mol
Mg+2H+===Mg2++H2↑
1 mol 1 mol
X mol Xmol
2Al+6H+===2Al3++3H2↑
2 mol 3 mol
Y mol 1.5 Y mol

(1)W(Al)=
 = 42.9%
= 42.9%(2)n(Mg): n(Al)="0." 3: 0. 2=3:2

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
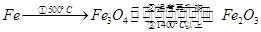
 H2O。
H2O。