��Ŀ����
����Ŀ������ͭ���仯�������ճ��������������Ź㷺��Ӧ�á���ش��������⣺
��1������Ԫ�����ڱ��е�λ����______����̬ͭԭ�ӵĺ�������Ų�ʽΪ______��
��2�������Fe(CO)x�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)x��������______![]() �������
�������![]() ��
��
��3��д��CO��һ�ֳ����ȵ�������ӵĽṹʽ______��������ȽϷе�ϸߵ�Ϊ______���ѧʽ����CN-��̼ԭ���ӻ��������Ϊ______��C��N��O��Ԫ�صĵ�һ����������Ϊ______![]() ��Ԫ�ط��ű�ʾ
��Ԫ�ط��ű�ʾ![]() ��
��
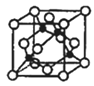
��4��ͭ������ͭԭ�ӵĶѻ���ʽ��ͼ��ʾ��

Mԭ�ӵļ۵����Ų�ʽΪ3s23p5��ͭ��M�γɻ�����ľ�����ͼ��ʾ���ڵ����ͭԭ�ӣ���
![]() �þ���Ļ�ѧʽΪ______��
�þ���Ļ�ѧʽΪ______��
![]() ��֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ���������______�����������������������������
��֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ���������______�����������������������������
![]() ��֪�þ�����ܶ�Ϊ
��֪�þ�����ܶ�Ϊ![]() gcm-3������٤������ΪNA����֪�þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ��Խ��ߵ�
gcm-3������٤������ΪNA����֪�þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ��Խ��ߵ�![]() ����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ______pm��ֻд����ʽ����
����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ______pm��ֻд����ʽ����
���𰸡��������ڵڢ��� 1s22s22p63s23p63d104s1��[Ar] 3d104s1 ���Ӿ��� ![]() CO sp�ӻ� N CuCl ����
CO sp�ӻ� N CuCl ���� 
��������
��1����Ϊ26��Ԫ�أ�������Ϊ26�����ݻ�̬ԭ�ӵĺ�������Ų�ʽ��ȷ���������ڱ��е�λ�ã�ͭΪ29��Ԫ�أ�������Ϊ29������д�����̬ԭ�ӵĺ�������Ų�ʽ��
��2�����Ӿ����۷е�ͣ�����״̬�²����磬�ۻ�ʱ���ƻ����ۼ���
��3��ԭ��������ȣ��۵�������ȵ����ӻ�Ϊ�ȵ����壬���ڷ��Ӿ��壬��Է����������ʱ������Խ�е�Խ�ߣ����ݼ۲���ӶԸ���ȷ��Cԭ�ӵ��ӻ���ʽ��
һ����˵�ǽ�����Խǿ����һ�����ܴ�
��4����Mԭ�ӵ���Χ�����Ų�ʽΪ3s23p5��˵��MΪ��Ԫ�أ���ͭ��M�γɻ�����ľ���ȷ���þ���Ļ�ѧʽ��
�ڵ縺�Բ�ֵ����1.7ԭ�Ӽ����γ����Ӽ���С��1.7ԭ�Ӽ��γɹ��ۼ���
�۽���������ֳ�8��С�����壬��þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���ΪС���������Խ��ߵ�һ�룬�ݴ˽��з�������
��1������Ԫ�ص���������֪������Ϊ26����ԭ�ӻ�̬ʱ�ĺ�������Ų�ʽΪ1s22s22p63s23p63d64s2��λ�����ڱ��е������ڵ�VIII�壻ͭΪ29��Ԫ�أ���̬ͭԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p63d104s1��[Ar] 3d104s1��
��2�������Fe(CO)x�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)x�������ڷ��Ӿ��壬�ʴ�Ϊ�����Ӿ��壻
��3��ԭ��������ȣ��۵�������ȵ����ӻ�Ϊ�ȵ����壬����CO��һ�ֳ����ȵ��������Ϊ�������ṹʽΪ![]() �����ڷ��Ӿ��壬��Է����������ʱ������Խ�е�Խ�ߣ�һ����̼�Ǽ��Է��ӣ������ǷǼ��Է��ӣ����Էе�ϸߵ�Ϊһ����̼��CN-��Cԭ�Ӽ۲���ӶԸ���=1+
�����ڷ��Ӿ��壬��Է����������ʱ������Խ�е�Խ�ߣ�һ����̼�Ǽ��Է��ӣ������ǷǼ��Է��ӣ����Էе�ϸߵ�Ϊһ����̼��CN-��Cԭ�Ӽ۲���ӶԸ���=1+![]() (4+1-1��3)=2�����Բ�ȡsp�ӻ���һ����˵�ǽ�����Խǿ����һ�����ܴ�����Ϊp����������ϵ���к�ǿ���ȶ��ԣ�N��p����������ǰ�����ģ�O��p���ʧȥһ�����Ӳ��ǰ�����ģ�����C��N��O��Ԫ�صĵ�һ����������ΪN��
(4+1-1��3)=2�����Բ�ȡsp�ӻ���һ����˵�ǽ�����Խǿ����һ�����ܴ�����Ϊp����������ϵ���к�ǿ���ȶ��ԣ�N��p����������ǰ�����ģ�O��p���ʧȥһ�����Ӳ��ǰ�����ģ�����C��N��O��Ԫ�صĵ�һ����������ΪN��
��4����ijMԭ�ӵ���Χ�����Ų�ʽΪ3s23p5����MΪ��Ԫ�أ���ͭ��M�γɻ�����ľ�����֪��4��ͭԭ�Ӷ��ھ����ڲ����ʸþ�����ͭ�ĸ���Ϊ4����ԭ�ӵĸ���Ϊ8��![]() +6��
+6��![]() =4�����Ըþ���Ļ�ѧʽΪ��CuCl��
=4�����Ըþ���Ļ�ѧʽΪ��CuCl��
�ڵ縺�Բ�ֵ����1.7ԭ�Ӽ����γ����Ӽ���С��1.7ԭ�Ӽ��γɹ��ۼ���ͭ��X�ĵ縺�Էֱ�Ϊ1.9��3.0����ֵΪ1.1С��1.7���γɹ��ۼ���
�۽���������ֳ�8��С�����壬��þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���ΪС���������Խ��ߵ�һ�룬1���þ����к���4��CuCl����þ�������Ϊ![]() �������ı߳�Ϊ
�������ı߳�Ϊ ��С������ı߳�Ϊ
��С������ı߳�Ϊ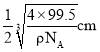 ��С���������Խ�����С������߳���
��С���������Խ�����С������߳���![]() ������С���������Խ���Ϊ
������С���������Խ���Ϊ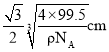 ����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ
����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ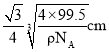 ����
����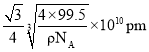 ��
��
�� | ͨʽ | �۵������� | ���幹�� |
CO2��SCN����NO | AX2 | 16e�� | ֱ���� |
CO | AX3 | 24e�� | ƽ�������� |
SO2��O3��NO | AX2 | 18e�� | V�� |
SO | AX4 | 32e�� | ���������� |
PO | AX3 | 26e�� | ������ |
CO��N2 | AX | 10e�� | ֱ���� |
CH4��NH | AX4 | 8e�� | ���������� |

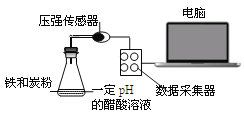
 ���㼤�������100�ִ��Ծ�ϵ�д�
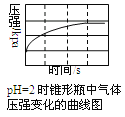
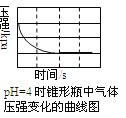
���㼤�������100�ִ��Ծ�ϵ�д�����Ŀ����������̼���������������ڲ�ͬ��ʵ�������½��з�Ӧ������ڲ�ͬʱ��(t)�ڲ����������(V)����������ͼ��ʾ������ͼʾ����ʵ������������˵����һ������ȷ������ ��
��� | ��Ӧ���� | c(HCl) / mol��L-1 | ��Ӧ�¶� / �� | ����״̬ | |
1 | a | 30 | ��ĩ״ | ||
2 | b | 30 | ��ĩ״ | ||
3 | c | 2.5 | ��״ | ||
4 | d | 2.5 | 30 | ��״ |
A. ��4��ʵ��ķ�Ӧ��������
B. ��1��ʵ���������Ũ�ȿ������
C. ��2��ʵ��������Ũ�ȿ��ܵ���2.5mol/L
D. ��3��ʵ��ķ�Ӧ�¶ȵ���30 ��
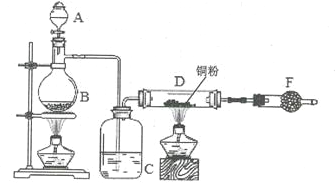
����Ŀ��������G�ĺϳ�·�����£�
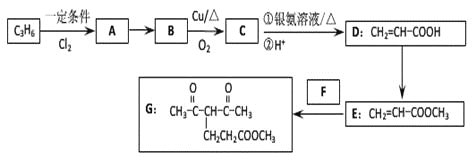
��1��D�к��������ŵ�����Ϊ_________��1 molG�����ӳɷ�Ӧ�������_______mol H2 ��
��2��ȥ��E�к�������D���Լ��Ͳ�����________________________________________�������й�E��˵����ȷ����_____________������ĸ��ţ���
A���ɷ���������Ӧ | B���������¿ɷ������۷�Ӧ |
C���ɷ���ȡ����Ӧ | D����CH2=CHCOOCH2CH3��ͬϵ�� |
E��������ˮ
��3��д��A��B�Ļ�ѧ��Ӧ����ʽ��________________________________________
��4����ӦE + F��G����������˶��ӳɷ�Ӧ���ͣ���F�Ľṹ��ʽΪ______________________��
��5����G��2����ԭ�ӵ����ʾ����������ʣ���дһ�ָ����ʵĽṹ��ʽ_________________��
����FeCl3��Һ����ɫ�� �������ϵ�һ��ȡ����ֻ��һ�֣�
��1mol������������2mol Na��1mol NaOH��