��Ŀ����
����Ŀ����ľ���и������Σ���Ҫ�ɷ���̼��أ������������Ȼ��غ�����ء��ִӲ�ľ������ȡ���Σ�����ʵ��������е�CO![]() ��SO
��SO![]() ��Cl����
��Cl����
��1���Ӳ�ľ������ȡ���ε�ʵ�����˳�����£��ٳ�����Ʒ�����ܽ��������________��������������ȴ�ᾧ��
��2���ڽ��Т����ܲ���ʱ����Ҫ�õ��������������÷ֱ��ǣ���________����________��
��3�����Ƶõ�������������Թܣ�������ˮ�ܽⲢ����Һ�ֳ����ݣ���װ��3֧�Թ��
���ڵ�һ֧�Թ������ϡ���ᣬ�ɹ۲���________���ɣ�֤����Һ����________���ӡ�
���ڵڶ�֧�Թ����������ϡ������ټ���BaCl2��Һ���ɹ۲쵽��________���ɣ�֤����Һ����________���ӡ�
���ڵ���֧�Թ����������Ba(NO3)2��Һ�����˺�������Һ�м�������ϡ������ټ���AgNO3��Һ���ɹ۲쵽��________���ɣ�֤����Һ����________���ӡ�
���𰸡� ���� ��������ֹ��Һ����©���� ���裬ʹ��Һ���Ⱦ��ȣ���ֹ��Һ�ɽ� ���� CO![]() ��ɫ���� SO
��ɫ���� SO![]() ��ɫ���� Cl��
��ɫ���� Cl��
����������1����ľ���еļ��ο�������ˮ���γ���Һ��Ȼ�����ù��˵ķ������Ի�ü��ε�ˮ��Һ�������õ�ˮ��Һ�����ᾧ���Ի���Ȼ��صĹ��壻��2���ڹ��˲����У������ò��������������������ᾧ�����У��ò����������裬ʹ��Һ���Ⱦ��ȣ���ֹ��Һ�ɽ�����4����̼��������ܺ����ᷴӦ���ɶ�����̼������Һ�м���ϡ���������������˵����̼������ӣ�����ԭ��Һ�еμ������ữ���Ȼ�����Һ���ų������������ӵĸ��ţ����������ɫ��������˵����Һ������������ӣ����ڵ���֧�Թ����������Ba(NO3)2��Һ�����˺�������Һ�м�������ϡ������ų����������ӵĸ��ţ��ټ���AgNO3��Һ��������ɫ������˵����Һ���������ӡ�

����Ŀ��������[(CH3COO)2Ni]��һ����Ҫ�Ļ���ԭ�ϡ�һ���Ժ�������(��NiS��Al2O3��FeO��CaO��SiO2)Ϊԭ��,��ȡ�������Ĺ�������ͼ���£�
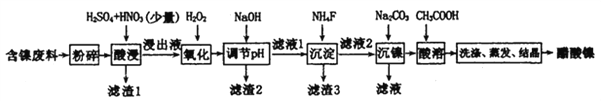
��������������������pH ��������ʵ��ܽ������±���
�������� | ��ʼ������pH | ������ȫ��pH | ���� | 20��ʱ�ܽ���(H2O) |
Fe3+ | 1.1 | 3.2 | CaSO4 | �� |
Fe2+ | 5.8 | 8.8 | NiF | ���� |
Al3+ | 3.0 | 5.0 | CaF2 | ���� |
Ni2+ | 6.7 | 9.5 | NiCO3 | Ksp=9.60��10-6 |
��1������pH ������,��ҺpH�ĵ��ڷ�Χ��________��
��2������1������3 ��Ҫ�ɷֵĻ�ѧʽ�ֱ���________��________��
��3��д�����������м���H2O2 ������Ӧ�����ӷ���ʽ________��
��4�����������,1molNiSʧȥ6 NA������,ͬʱ����������ɫ�ж����塣д���÷�Ӧ�Ļ�ѧ����ʽ________��
��5������������,��c(Ni2+)=2.0 mol��L-1,��ʹ100 mL����Һ�е�Ni2+������ȫ[c(Ni2+)��10-5mol��L-1]������Ҫ����Na2CO3�������������Ϊ________g��(����С�����1λ��Ч����)
��6������������������,�ڲ�ͬ�¶��¶Ժ������Ͻ������������������ʱ��仯��ͼ�����������¶���ʱ��ֱ�Ϊ________��

����Ŀ������500 mL 0.25 mol��L��1��NaOH��Һ���Իش��������⣺
��1�����㣺��ҪNaOH���������Ϊ_______________��
��2��ijѧ����������ƽ����һ��С�ձ�������������ǰ��������ڱ�ߵ���̶ȴ�����ƽ��ֹʱ����ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�________(��������������������)�ұߵ����̡���ʹ��ƽƽ�⣬�����еIJ���Ϊ_________________________________��
�ٶ����ճƵ�С�ձ�������Ϊ______(����32.6 g������31.61 g��)��������������ʾ�������Ϸ������룬��������ʾ��������ȡ������ij������̣����ڱ���ϻ��������λ��(����������ʾ)��
��������/g | 50 | 20 | 20 | 10 | 5 |
����(ȡ���� �����) |
![]()
____________
��3�����Ʒ�����ijͬѧ������ĸ��������裺
����ʢ��NaOH���ձ��м���200 mL����ˮʹ���ܽ⣬����ȴ�����¡�
�ڽ�NaOH��Һ�ز�����ע��________�С�
�ۼ���������ƿ�м�����ˮ��Һ��ӽ��̶���1��2 cm��
�ܸ��ý�ͷ�ιܼ�����ˮ���̶��ߣ��Ӹ�ҡ�ȡ�
������������������Һ���ʵ���Ũ�Ȼ�________(����ƫ������ƫ����������Ӱ����)������Ӱ�����治����д������Ӱ�죬Ӧ�ڲ���________�Ͳ���________֮�䣬����IJ�����____________��
����Ŀ�����������һ�ֵ��͵�ǿ��������������ʵ���һ����ڻ��������ж�����Ҫ��Ӧ�á���ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)��

��1���Ʊ�����ѡ�õ�ҩƷΪ:������غ�Ũ���ᣬ��Ӧ�����ӷ���ʽΪ:______________________��
��2��װ��B��������_______________________�����B�г���©��Һ����������˵��____________________________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η���________��(ѡa��b��c)
a | b | c | |
I | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
II | ��ʯ�� | Ũ���� | ��ˮ�Ȼ��� |
III | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ����ɫ��˵�����ķǽ����Դ����塣��������D�е�������Һ����E�У���E���۲쵽��������_______________________________��������______(��ܡ����ܡ�)˵����ķǽ�����ǿ�ڵ⣬ԭ����______________________________________��