��Ŀ����
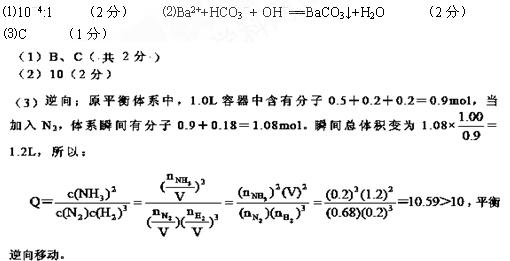
(15��)A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬AԪ�ص�ԭ�Ӱ뾶��С��BԪ��ԭ�ӵ��������������ڲ��������2����CԪ�ص�����������ˮ����X�����⻯�ﷴӦ����һ����Y��A��B��C��E����Ԫ�ض�����DԪ���γ�ԭ�Ӹ����Ȳ���ͬ�ij���������ش��������⣺
�ų����£�X��Y��ˮ��Һ��pH��Ϊ5��������ˮ��Һ����ˮ�������H+Ũ��֮���� ��
��A��B��D��E����Ԫ����ɵ�ij������������ֽ⡣д�������û�������Һ��������Ba(OH)2��Һ��Ӧ�����ӷ���ʽ ��
��A��B��D��E����Ԫ����ɵ�ij��������Ũ��Ϊ0.1 mol/Lʱ��pH��ӽ� ��
A��5.6 B��7.0 C��8.4 D��13.0
����һ��װ�п��ƶ������������н������·�Ӧ��C2��g��+3A2��g�� 2CA3��g��
2CA3��g��
��H����92.4 kJ��mol��1.��Ӧ�ﵽƽ���������к���C2��0.5mol��A2��0.2mol��CA3��0.2 mol�����ݻ�Ϊ1 .0 L��
.0 L��
�������ɴ�ƽ��ǰ��������ʼ���������¼��ֿ��ܣ����в���������________��
A��C2��0.6mol��A2��0.5mol��CA3��0mol
B��C2��0mol��A2��0mol��CA3��1.2mol
C��C2��0.6mol/L��A2��0.5mol/L��CA3��0.2mol/L
�������ƽ����ϵ��ƽ�ⳣ��K��________��
����������¶Ⱥ�ѹǿ���䣬������ƽ����ϵ�м���0.18molC2��ƽ�⽫________������������������ƶ���������
��
����֪0.4mol Һ̬C2 A4��������Һ̬˫��ˮ��Ӧ������C2��ˮ�������ų�256.65 kJ
��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��

����

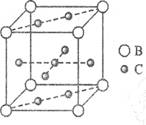
 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�

 2CA3��g��
2CA3��g��  B
B D
D Bת���Ļ�ѧ����ʽ��
��
Bת���Ļ�ѧ����ʽ��
��