��Ŀ����
��8�֣�A��E����Ԫ���У���A��B��������Ϊ������Ԫ�����ǵ����ʻ�ԭ�ӽṹ���±���
��1��C������ǿ����Һ��Ӧ�����ӷ���ʽ��
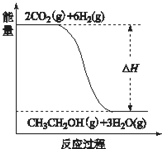
��2�����ڸ��������£���D��E��ɵ���̬��������A�ķ�ĩ��ַ�Ӧ������8gE�ĵ��ʺ���Ӧ�Ĺ�̬������ʱ���ų�a KJ�������˷�Ӧ���Ȼ�ѧ����ʽ��
��A��ij���Ȼ�����Һ�ʻ�ɫ��������ͨ������SO2���壬��Һ��Ϊdz��ɫ���˷�Ӧ�����ӷ���ʽ��
��3����2mL 0.5mol/L��ACl3��Һ�м���3mL 3mol/L KF��Һ��ACl3��Һ�ʳ���ɫ���ټ���KI��Һ��CCl4���ã�CCl4�㲻��ɫ��������˵����ȷ���ǣ���֪������Fe3+>I2��
������ĸ����
a. A3+����I-������Ӧ b. A3+��F-������ɲ���I-��Ӧ������
c. F-ʹI-�������Լ��� d. A3+��F-��ԭΪA2+��ʹ��Һ�в��ٴ���A3+
| Ԫ�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ����Ϊ�����г����Ľ������ý��������ֺ�ɫ���������һ���д��ԣ�����Է����������160 |
| B | ����Ϊ�����г����Ľ����������ԭ��������A��8 |
| C | �ؿ��к������Ľ���Ԫ�� |
| D | Ԫ�ص���������������������������3�� |
| E | E��ij��ԭ�ӵ�ԭ�Ӻ���û������ |
��2�����ڸ��������£���D��E��ɵ���̬��������A�ķ�ĩ��ַ�Ӧ������8gE�ĵ��ʺ���Ӧ�Ĺ�̬������ʱ���ų�a KJ�������˷�Ӧ���Ȼ�ѧ����ʽ��
��A��ij���Ȼ�����Һ�ʻ�ɫ��������ͨ������SO2���壬��Һ��Ϊdz��ɫ���˷�Ӧ�����ӷ���ʽ��
��3����2mL 0.5mol/L��ACl3��Һ�м���3mL 3mol/L KF��Һ��ACl3��Һ�ʳ���ɫ���ټ���KI��Һ��CCl4���ã�CCl4�㲻��ɫ��������˵����ȷ���ǣ���֪������Fe3+>I2��
������ĸ����
a. A3+����I-������Ӧ b. A3+��F-������ɲ���I-��Ӧ������
c. F-ʹI-�������Լ��� d. A3+��F-��ԭΪA2+��ʹ��Һ�в��ٴ���A3+
��1��2Al+2OH-+2H2O=2AlO2-+3H2����2�֣�
��2����3Fe(s)+4H2O(g) Fe3O4(s)+4H2(g) ��H=-akJ/mol��2�֣�
Fe3O4(s)+4H2(g) ��H=-akJ/mol��2�֣�
��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��2�֣� ��3��b ��1�֣�
��2����3Fe(s)+4H2O(g)
 Fe3O4(s)+4H2(g) ��H=-akJ/mol��2�֣�
Fe3O4(s)+4H2(g) ��H=-akJ/mol��2�֣���2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��2�֣� ��3��b ��1�֣�
����Ԫ�صĽṹ�����ʿ�֪��A��Fe��B��Cu��C��Al��D��O��E��H��
��1�����ܺ�����������Һ��Ӧ����ƫ�����ƺ�����������ʽΪ
2Al+2OH-+2H2O=2AlO2-+3H2����
��2����D��E��ɵ���̬��������ˮ������8gE�ĵ��ʷų�a KJ�����������Ȼ�ѧ����ʽΪ3Fe(s)+4H2O(g) Fe3O4(s)+4H2(g) ��H=-akJ/mol��
Fe3O4(s)+4H2(g) ��H=-akJ/mol��
���Ȼ������������ԣ��ܰ�SO2�����������ᣬ����ʽΪ2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
��3������KI��Һ��CCl4���ã�CCl4�㲻��ɫ��˵��û�е��ʵ����ɣ��������Ӻ�F����������˲���I-��Ӧ�����ʣ����Դ�ѡb��
��1�����ܺ�����������Һ��Ӧ����ƫ�����ƺ�����������ʽΪ
2Al+2OH-+2H2O=2AlO2-+3H2����
��2����D��E��ɵ���̬��������ˮ������8gE�ĵ��ʷų�a KJ�����������Ȼ�ѧ����ʽΪ3Fe(s)+4H2O(g)
 Fe3O4(s)+4H2(g) ��H=-akJ/mol��
Fe3O4(s)+4H2(g) ��H=-akJ/mol�����Ȼ������������ԣ��ܰ�SO2�����������ᣬ����ʽΪ2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
��3������KI��Һ��CCl4���ã�CCl4�㲻��ɫ��˵��û�е��ʵ����ɣ��������Ӻ�F����������˲���I-��Ӧ�����ʣ����Դ�ѡb��

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ