��Ŀ����
����Ŀ�����������Ҷ��ᣬ�㷺������ֲ��ԴʳƷ�У�������;�dz��㷺���ⶨij���ᾧ�壨���ΪH2C2O4��nH2O����H2C2O4��������������������ʵ�飺��ȡ w g ���ᾧ����� 250mL ��Һ����ȡ 25.00mL ���������Һ������ƿ�У� ��������ϡH2SO4 ������ƿ�ײ���һ�Ű�ֽ����Ũ��Ϊ 0.100mol��L-1KMnO4 ��Һ�ζ����ζ�ʱ���������ķ�ӦΪ��2KMnO4��5H2C2O4��3H2SO4��K2SO4��10CO2����2MnSO4��8H2O��
��ش��������⣺
(1)������Ӧ�Ļ�ԭ����_____���ѧʽ����
(2)��������Ϊ����ȷŨ�ȵIJ�����Һ�������õ��������У���ƽ�������룩���ձ���ҩ�ס�250ml ����ƿ��___________ ��_______________��
(3)����ƿ�ײ���һ�Ű�ֽ��������_____��
(4)�ζ���Ӧѡ��_______ʽ�ζ��ܣ����������������������жϵζ������ı���_________��
(5)���εζ����� KMnO ��Һ���ƽ��Ϊ 20.00mL����ʵ�������������Һ�����ʵ���Ũ��Ϊ______________mol��L-1�����ᾧ���� H2C2O4 ����������Ϊ_______________����ʵ������У���ƿ������ˮϴ��֮�����ò�����Һ��ϴ���Բⶨ�����ɵ�Ӱ����_______������ƫ��������ƫС��������Ӱ�죩
���𰸡�H2C2O4 ��ͷ�ι� ������ ���ڹ۲���ɫ�仯��ȷ�ж��ζ��յ� �ᡢ���������һ��KMnO4��Һʱ����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ԭ 0.2mol/L ![]() ��100% . ƫ��
��100% . ƫ��
��������
(1) ��ԭ��������Ԫ�صĻ��ϼ����ߣ�����������Ӧ��
(2)��������һ�����ʵ���Ũ����Һ�IJ�������ȷ������Ҫ�IJ���������
(3) ������ƿ�µ�һ�Ű�ֽʹ�ζ��յ���ɫ�仯�����ԣ����ڷֱ棻
(4) ����KMnO4 ��Һ��ǿ�����ԣ��ζ��յ�����μ�����KMnO4��Һ����Һ����ɫ��
(5) ���ݹ�ϵʽ2KMnO4��5H2C2O4���������Һ��c(H2C2O4)���ټ�����ᾧ���� H2C2O4 ����������������c(����)=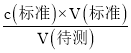 ��������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
��������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
(1) ��Ӧ2KMnO4��5H2C2O4��3H2SO4��K2SO4��10CO2����2MnSO4��8H2O��̼Ԫ�صĻ��ϼ۴�+3������Ϊ+4�ۣ�����������Ӧ������Ӧ�Ļ�ԭ����H2C2O4��
(2) ʵ�����Ϊ������ȷŨ�ȵIJ�����Һ250.00mL������Ҫ��ʵ��������Ҫ����ƽ(������)��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܵȣ��ʻ���Ҫ�������ͽ�ͷ�ιܣ�
(3) ����ƿ�µ�һ�Ű�ֽʹ�ζ��յ���ɫ�仯�����ԣ����ڹ۲���ɫ�仯��ȷ�ж��ζ��յ㣻
(4) KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��ʽ�ζ����е���Ƥ�ܣ���KMnO4��ҺӦװ����ʽ�ζ����У�KMnO4��Һ����ɫ�����ᷴӦ��ϣ����������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ㣻
(5) ���ݹ�ϵʽ2KMnO4��5H2C2O4��֪25.0mL������Һ��n(H2C2O4)=![]() ��0.100mol��L-1��20��10-3mol=0.005mol��c(H2C2O4)=
��0.100mol��L-1��20��10-3mol=0.005mol��c(H2C2O4)=![]() =0.2mol/L�����ᾧ���� H2C2O4 ����������Ϊ
=0.2mol/L�����ᾧ���� H2C2O4 ����������Ϊ =
=![]() ��100%����ƿ������ˮϴ��֮���ò�����Һ��ϴ������Һ�����ʵ���ƫ�����v(��)����c(����)=
��100%����ƿ������ˮϴ��֮���ò�����Һ��ϴ������Һ�����ʵ���ƫ�����v(��)����c(����)=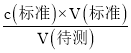 ��֪����֪c(����)ƫ��
��֪����֪c(����)ƫ��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�������±��ṩ������![]() �г��������Լ���ѡ
�г��������Լ���ѡ![]() ���ܴﵽ��Ӧʵ��Ŀ��һ����
���ܴﵽ��Ӧʵ��Ŀ��һ����
ѡ�� | ʵ��Ŀ�� | ���� |
A | ��CCl4��ȡ��ˮ�е��嵥�� | �ձ�������������Һ©�� |
B | ��ʳ��ˮ�л��NaCl���� | �ƾ��ơ��������������������� |
C | ����100mL1.0mol/L��NaOH��Һ | ҩ�ס���ƽ����Ͳ���ձ�������������ͷ�ιܡ�100mL������ƿ |
D | �������ȥBaSO4�л�������BaCO3 | ©�����ձ�������������ͷ�ιܡ���ֽ |
A. B. C. D.
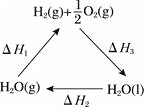
����Ŀ����2 L�ܱ�������,800 ��ʱ��Ӧ2NO(g)+O2(g)![]() 2NO2(g)����H<0,n(NO)��ʱ��ı仯���±�:
2NO2(g)����H<0,n(NO)��ʱ��ı仯���±�:
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
(1)��ͼ�б�ʾNO2�ı仯��������_____����O2��ʾ��0~2 s�ڸ÷�Ӧ��ƽ������v=____��

(2)��˵���÷�Ӧ�Ѵﵽƽ��״̬����____��
A.v(NO2)=2v(O2) B.������ѹǿ���ֲ���
C.v��(NO)=2v��(O2) D.�������ܶȱ��ֲ���