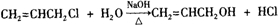��Ŀ����
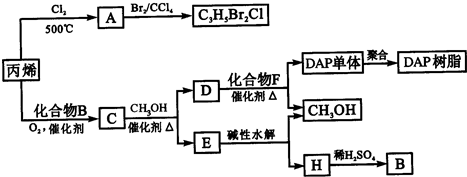
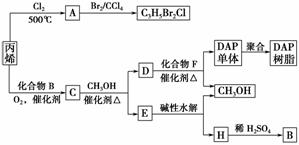
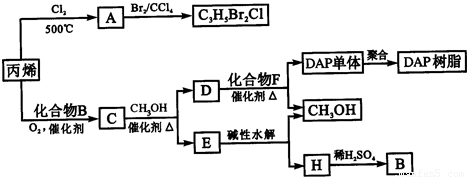
��ϩ�����ںϳ�ɱ�������߳��ũҩ������ʽΪC3H5Br2Cl����Ӧ�ù㷺��DAP��֬��

��֪���봼�ɷ���������������Ӧ��
RCOOR��+R��OH
RCOOR��+R��OH ��R��R�䡢R�����������
��1��ũҩ����C3H5Br2Cl��ÿ��̼ԭ���Ͼ�����±ԭ�ӣ�
��A�Ľṹ��ʽ�� ��A���еĹ����������� ��
���ɱ�ϩ����A�ķ�Ӧ������ ��
��2��Aˮ��ɵõ�D����ˮ�ⷴӦ�Ļ�ѧ����ʽ�� ��
��3��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%��C�Ľṹ��ʽ�� ��
��4������˵����ȷ���ǣ�ѡ�������ĸ�� ��
a��C�ܷ����ۺϷ�Ӧ����ԭ��Ӧ��������Ӧ
b��C����2������������ͬ���칹����4��
c��D������IJ�����B������ͬ����Է�������
d��E���з�����ζ���������Ҵ�
��5��E��ˮ����ᆳ�������յõ��״���B�����߾���ѭ��������DAP��֬���Ʊ������н��״���H����IJ��������� ��
��6��F�ķ���ʽΪC10H10O4��DAP����Ϊ���Ķ�Ԫȡ���������ȡ���������ڶ�λ���õ��屽���ϵ�һ��ȡ����ֻ�����֣�D��F��Ӧ����DAP����Ļ�ѧ����ʽ�� ��

��֪���봼�ɷ���������������Ӧ��
RCOOR��+R��OH
| ���� | �� |
��1��ũҩ����C3H5Br2Cl��ÿ��̼ԭ���Ͼ�����±ԭ�ӣ�
��A�Ľṹ��ʽ��
���ɱ�ϩ����A�ķ�Ӧ������
��2��Aˮ��ɵõ�D����ˮ�ⷴӦ�Ļ�ѧ����ʽ��
��3��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%��C�Ľṹ��ʽ��
��4������˵����ȷ���ǣ�ѡ�������ĸ��
a��C�ܷ����ۺϷ�Ӧ����ԭ��Ӧ��������Ӧ
b��C����2������������ͬ���칹����4��
c��D������IJ�����B������ͬ����Է�������
d��E���з�����ζ���������Ҵ�
��5��E��ˮ����ᆳ�������յõ��״���B�����߾���ѭ��������DAP��֬���Ʊ������н��״���H����IJ���������
��6��F�ķ���ʽΪC10H10O4��DAP����Ϊ���Ķ�Ԫȡ���������ȡ���������ڶ�λ���õ��屽���ϵ�һ��ȡ����ֻ�����֣�D��F��Ӧ����DAP����Ļ�ѧ����ʽ��
������ũҩ����C3H5Br2Cl��ÿ��̼ԭ���Ͼ�����±ԭ�ӣ����֪��ϩ��500�������·���ȡ����Ӧ����
A��CH2=CHCH2Cl����A���巢���ӳɷ�Ӧ����C3H5Br2Cl��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%������Է�������Ϊ6.25��16=100�������к�N��C��=
=5��N��H��=
=8��N��O��=
=2�������ʽΪC5H8O2��C�ܼ״�������������ӦΪ������ϩ��B��Ӧ���Եõ�C����C�Ľṹ��ʽΪCH3COOCH2CH=CH2��C��D������������Ӧ����DΪHOCH2CH=CH2��EΪCH3COOCH3��ˮ�����ɼ״���B����BΪCH3COOH��HΪCH3COONa���Դ˽���л�������������
A��CH2=CHCH2Cl����A���巢���ӳɷ�Ӧ����C3H5Br2Cl��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%������Է�������Ϊ6.25��16=100�������к�N��C��=
| 100��60% |
| 12 |
| 100��8% |
| 1 |
| 100��32% |
| 16 |
����⣺ũҩ����C3H5Br2Cl��ÿ��̼ԭ���Ͼ�����±ԭ�ӣ����֪��ϩ��500�������·���ȡ����Ӧ����
A��CH2=CHCH2Cl����A���巢���ӳɷ�Ӧ����C3H5Br2Cl��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%������Է�������Ϊ6.25��16=100�������к�N��C��=
=5��N��H��=
=8��N��O��=
=2�������ʽΪC5H8O2��C�ܼ״�������������ӦΪ������ϩ��B��Ӧ���Եõ�C����C�Ľṹ��ʽΪCH3COOCH2CH=CH2��C��D������������Ӧ����DΪHOCH2CH=CH2��EΪCH3COOCH3��ˮ�����ɼ״���B����BΪCH3COOH��HΪCH3COONa��
��1���������Ϸ�����֪AΪCH2=CHCH2Cl������̼̼˫������ԭ�ӹ����ţ��ʴ�Ϊ��CH2=CHCH2Cl��̼̼˫������ԭ�ӣ�
���ɱ�ϩ����A�ķ�Ӧ������ȡ�����ʴ�Ϊ��ȡ����
��2��Aˮ��ɵõ�D����ˮ�ⷴӦ�Ļ�ѧ����ʽΪCH2=CHCH2Cl+H2O
HOCH2CH=CH2+HCl��
�ʴ�Ϊ��CH2=CHCH2Cl+H2O
HOCH2CH=CH2+HCl��
��3��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%������Է�������Ϊ6.25��16=100�������к�N��C��=
=5��N��H��=
=8��N��O��=
=2�������ʽΪC5H8O2��C�ܼ״�������������ӦΪ������ϩ��B��Ӧ���Եõ�C����C�Ľṹ��ʽΪCH3COOCH2CH=CH2��
�ʴ�Ϊ��CH3COOCH2CH=CH2��
��4��a��CΪCH3COOCH2CH=CH2���ɷ����ۺϷ�Ӧ����ԭ��Ӧ��������Ӧ����a��ȷ��
b��C����2������������ͬ���칹����CH3C��CH3��=CH2COOH��CH3CH=C��CH3��COOH2�֣���b����
c��DΪCH2=CHCH2OH���ӳɲ���ΪCH3CH2CH2OH����Է�������Ϊ60��BΪCH3COOH����Է�������Ϊ60����c��ȷ��
d��EΪCH3COOCH3�����з�����ζ���������Ҵ�����d��ȷ��
�ʴ�Ϊ��a c d��
��5��HΪ�����ƣ���״��ķе����ϴ���������ķ��������룬�ʴ�Ϊ������
��6��F�ķ���ʽΪC10H10O4��DAP����Ϊ���Ķ�Ԫȡ���������ȡ���������ڶ�λ���õ��屽���ϵ�һ��ȡ����ֻ�����֣�Ӧλ����λ��DΪCH2=CHCH2OH����FΪ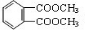 ����Ӧ����DAP����Ļ�ѧ����ʽ��
����Ӧ����DAP����Ļ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
A��CH2=CHCH2Cl����A���巢���ӳɷ�Ӧ����C3H5Br2Cl��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%������Է�������Ϊ6.25��16=100�������к�N��C��=
| 100��60% |
| 12 |
| 100��8% |
| 1 |
| 100��32% |
| 16 |
��1���������Ϸ�����֪AΪCH2=CHCH2Cl������̼̼˫������ԭ�ӹ����ţ��ʴ�Ϊ��CH2=CHCH2Cl��̼̼˫������ԭ�ӣ�
���ɱ�ϩ����A�ķ�Ӧ������ȡ�����ʴ�Ϊ��ȡ����
��2��Aˮ��ɵõ�D����ˮ�ⷴӦ�Ļ�ѧ����ʽΪCH2=CHCH2Cl+H2O
| NaOH |
| �� |
�ʴ�Ϊ��CH2=CHCH2Cl+H2O
| NaOH |
| �� |
��3��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����C�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%������Է�������Ϊ6.25��16=100�������к�N��C��=
| 100��60% |
| 12 |
| 100��8% |
| 1 |
| 100��32% |
| 16 |
�ʴ�Ϊ��CH3COOCH2CH=CH2��
��4��a��CΪCH3COOCH2CH=CH2���ɷ����ۺϷ�Ӧ����ԭ��Ӧ��������Ӧ����a��ȷ��
b��C����2������������ͬ���칹����CH3C��CH3��=CH2COOH��CH3CH=C��CH3��COOH2�֣���b����
c��DΪCH2=CHCH2OH���ӳɲ���ΪCH3CH2CH2OH����Է�������Ϊ60��BΪCH3COOH����Է�������Ϊ60����c��ȷ��
d��EΪCH3COOCH3�����з�����ζ���������Ҵ�����d��ȷ��
�ʴ�Ϊ��a c d��
��5��HΪ�����ƣ���״��ķе����ϴ���������ķ��������룬�ʴ�Ϊ������
��6��F�ķ���ʽΪC10H10O4��DAP����Ϊ���Ķ�Ԫȡ���������ȡ���������ڶ�λ���õ��屽���ϵ�һ��ȡ����ֻ�����֣�Ӧλ����λ��DΪCH2=CHCH2OH����FΪ
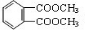 ����Ӧ����DAP����Ļ�ѧ����ʽ��
����Ӧ����DAP����Ļ�ѧ����ʽ�� ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶ���ϳɣ�ע��������ʵĽṹ����Ӧ�������ƶϣ�ע������ŵ�������ת������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ