��Ŀ����
��12�֣���ˮ�д�������ƽ�⣺Cl2 +H2O ? HCl+HClO������ˮ�м����������ʣ��ش�����⣺
��1����������CaCO3(s)��������Ӧ�����ӷ���ʽ��ʾΪ__________________��ƽ����________�ƶ��������������������ͬ����HClO��Ũ��______�����������С�����䡱����ͬ����
��2����������NaCl(s)��ƽ����_________�ƶ���HClO��Ũ��___________��
��3����������NaOH(s)��ƽ����__________�ƶ���HClO��Ũ��____________��
��4����������NaBr��Һ��������Ӧ�����ӷ���ʽ��ʾΪ____��HCl�����ʵ���_____��
��5����������ŨƷ����Һ��ʵ������Ϊ_____________��HCl�����ʵ���___________��
��6��������ˮϡ�ͣ�HClO��Ũ��__________��
��1����������CaCO3(s)��������Ӧ�����ӷ���ʽ��ʾΪ__________________��ƽ����________�ƶ��������������������ͬ����HClO��Ũ��______�����������С�����䡱����ͬ����
��2����������NaCl(s)��ƽ����_________�ƶ���HClO��Ũ��___________��
��3����������NaOH(s)��ƽ����__________�ƶ���HClO��Ũ��____________��
��4����������NaBr��Һ��������Ӧ�����ӷ���ʽ��ʾΪ____��HCl�����ʵ���_____��
��5����������ŨƷ����Һ��ʵ������Ϊ_____________��HCl�����ʵ���___________��
��6��������ˮϡ�ͣ�HClO��Ũ��__________��
��CaCO3+2H+=Ca2++CO2��+2H2O���������������١��������١���Cl2+2Br��=Br2+2Cl�������١���Ʒ����Һ��ɫ�����ʼ��١�
��

��ϰ��ϵ�д�
�����Ŀ

 �����ʵ���֮��Ϊ1��1��д�����ӷ���ʽ��
�����ʵ���֮��Ϊ1��1��д�����ӷ���ʽ�� ������ͼ�������𣿡�
������ͼ�������𣿡�
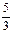 ��36��5g
��36��5g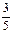 ��36��5g
��36��5g