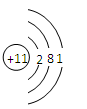��Ŀ����
���ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ��Ư���dz��õ���������
(1) ��ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ��Ӧ����ʽΪ ����2�֣�
(2)Ư�۵���Ч�ɷ��ǣ��ѧʽ�� ����1�֣�
(3)Ư������ˮ���ܿ����е�CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬ��ѧ��Ӧ����ʽΪ ����2�֣�
��4������������ ��1�֣� �Ĺ��壬����ˮ�Ͷ�����̼��Ӧ�Ļ�ѧ����ʽ����Ϊ ����2�֣� ��2�֣���ˣ��������ƿ���Ϊ �� ����������Դ����1�֣�
2 ����6�֣���20g�ռ����Ƴ�500mL��Һ�������ʵ���Ũ�ȣ�Ϊ mol/L����2�֣�����ȡ��1mL�������ʵ���Ũ��Ϊ mol/L����2�֣�������1mL��Һ��ˮϡ�͵�100mL��������Һ�����ʵ����ʵ���Ũ��Ϊ mol/L����2�֣�
(1) ��ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ��Ӧ����ʽΪ ����2�֣�
(2)Ư�۵���Ч�ɷ��ǣ��ѧʽ�� ����1�֣�
(3)Ư������ˮ���ܿ����е�CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬ��ѧ��Ӧ����ʽΪ ����2�֣�
��4������������ ��1�֣� �Ĺ��壬����ˮ�Ͷ�����̼��Ӧ�Ļ�ѧ����ʽ����Ϊ ����2�֣� ��2�֣���ˣ��������ƿ���Ϊ �� ����������Դ����1�֣�
2 ����6�֣���20g�ռ����Ƴ�500mL��Һ�������ʵ���Ũ�ȣ�Ϊ mol/L����2�֣�����ȡ��1mL�������ʵ���Ũ��Ϊ mol/L����2�֣�������1mL��Һ��ˮϡ�͵�100mL��������Һ�����ʵ����ʵ���Ũ��Ϊ mol/L����2�֣�
1.����12�֣�(1)2Cl2 + 2 Ca(OH)2 ="=" Ca(ClO)2 + CaCl2 + 2H2O
(2��Ca(ClO)2
(3��Ca(ClO)2 + CO2 + H2O ="=" CaCO3��+ 2 HClO
(4������ɫ 2Na2O2 + 2 H2O ="=" 4NaOH + O2�� 2Na2O2 + 2 CO2 ="=" 2Na2CO3 + O2 ������� DZˮͧ
2)��6�֣� 1 1 0.01
(2��Ca(ClO)2
(3��Ca(ClO)2 + CO2 + H2O ="=" CaCO3��+ 2 HClO
(4������ɫ 2Na2O2 + 2 H2O ="=" 4NaOH + O2�� 2Na2O2 + 2 CO2 ="=" 2Na2CO3 + O2 ������� DZˮͧ
2)��6�֣� 1 1 0.01
��

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ