��Ŀ����
��1���ϳɰ���ӦN2��g��+3H2��g��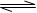 2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ��1mol N2��3mol H2��ƽ���NH3���������Ϊa�����ں��¡�������������ƽ����ϵ��ͨ��lmol N2��3mol H2��ƽ���NH3���������Ϊb����a b������ڡ��������ڡ�����С�ڡ�����
2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ��1mol N2��3mol H2��ƽ���NH3���������Ϊa�����ں��¡�������������ƽ����ϵ��ͨ��lmol N2��3mol H2��ƽ���NH3���������Ϊb����a b������ڡ��������ڡ�����С�ڡ�����
��2����һ���п̶ȵ�������һ������ĸ�Ĥ�ֳ����������֣�����ͼ��ʾ��
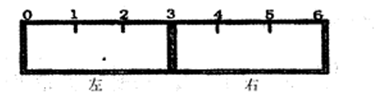
���ø�ĤΪ�����ӽ���Ĥ��ֻ��������������ͨ�������ҽ���Ĥ�̶���3������߳�����������KSCN��Һ��FeCl2��Һ���ұ߳�������KMnO4��Һ��һ��ʱ���ɹ۲쵽�������� ���� ����������ѡ����ѡ��
��д���ұ߲�������������ӷ���ʽ ��
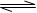 2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ��1mol N2��3mol H2��ƽ���NH3���������Ϊa�����ں��¡�������������ƽ����ϵ��ͨ��lmol N2��3mol H2��ƽ���NH3���������Ϊb����a b������ڡ��������ڡ�����С�ڡ�����
2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ��1mol N2��3mol H2��ƽ���NH3���������Ϊa�����ں��¡�������������ƽ����ϵ��ͨ��lmol N2��3mol H2��ƽ���NH3���������Ϊb����a b������ڡ��������ڡ�����С�ڡ�������2����һ���п̶ȵ�������һ������ĸ�Ĥ�ֳ����������֣�����ͼ��ʾ��

���ø�ĤΪ�����ӽ���Ĥ��ֻ��������������ͨ�������ҽ���Ĥ�̶���3������߳�����������KSCN��Һ��FeCl2��Һ���ұ߳�������KMnO4��Һ��һ��ʱ���ɹ۲쵽�������� ���� ����������ѡ����ѡ��
| A���������� | B����Һ��Ϊ��ɫ | C����Һ��ɫ��dz | D���к��ɫ�������� |
��1�����ڣ�2�֣���2��A��2�֣� C��2�֣�
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��2�֣�
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��2�֣�
��1����Ӧ��������У����¡���ѹ�����£�ƽ��ʱ�����ԭ��ҪС����ʱNH3���������Ϊa�����ں��¡����������£��൱���ں�ѹ�����½��м�ѹ��������ѹƽ���������ƶ���ƽ���NH3���������Ҫ��С��bС��a��
��2�������ӽ���Ĥ��ֻ��������������Ĥ����������Ҷˣ�ͬʱ����Fe2+��MnO4-���Ӽ��������ԭ��Ӧ���������ӱ�����Ϊ���������ӣ������������ԭΪ���������ӡ������Һ��ɫ����ûӰ�죬�Ҷ���Һ��ɫ��dz��
�����������Чƽ�⣬������ԭ��Ӧ�����֪ʶ��
��2�������ӽ���Ĥ��ֻ��������������Ĥ����������Ҷˣ�ͬʱ����Fe2+��MnO4-���Ӽ��������ԭ��Ӧ���������ӱ�����Ϊ���������ӣ������������ԭΪ���������ӡ������Һ��ɫ����ûӰ�죬�Ҷ���Һ��ɫ��dz��
�����������Чƽ�⣬������ԭ��Ӧ�����֪ʶ��

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
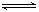 2Z(g)�ﻯѧƽ��״̬�ı�־��
2Z(g)�ﻯѧƽ��״̬�ı�־�� x Z(g) ��H��0
x Z(g) ��H��0 2 NH3 ��ÿ����2 mol NH3�ų�92.4 kJ�����������ں��º�ѹ���ܱ������н�������ʵ�飺��ͨ��1 mol N2��3 mol H2����ƽ��ʱ�ų�����ΪQ1����ͨ��2 mol N2��6 mol H2����ƽ��ʱ�ų�����ΪQ2�������й�ϵ��ȷ����
2 NH3 ��ÿ����2 mol NH3�ų�92.4 kJ�����������ں��º�ѹ���ܱ������н�������ʵ�飺��ͨ��1 mol N2��3 mol H2����ƽ��ʱ�ų�����ΪQ1����ͨ��2 mol N2��6 mol H2����ƽ��ʱ�ų�����ΪQ2�������й�ϵ��ȷ���� 2SO3(g)����H=��akJ��mo1��2����ͬ������Ҫ��õ�2akJ��������������ʵ����ʵ���������( )
2SO3(g)����H=��akJ��mo1��2����ͬ������Ҫ��õ�2akJ��������������ʵ����ʵ���������( ) �������ͼʾ�ش��������⣺
�������ͼʾ�ش��������⣺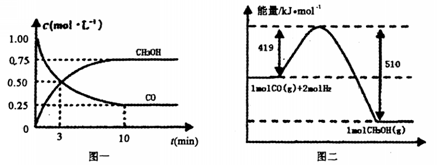
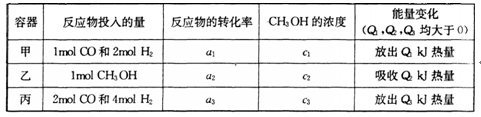
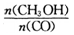 �������________________
�������________________
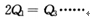
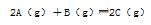 �������ڿ�ʼ�������и������ʣ��ﵽƽ��ʱ�淴Ӧ������С����
�������ڿ�ʼ�������и������ʣ��ﵽƽ��ʱ�淴Ӧ������С����