��Ŀ����
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺

��1����Ũ��������ʵ���Ũ��Ϊ__________mol/L��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ�� ���Ķ��ٶ��仯����__________��
A����Һ��H2SO4�����ʵ��� B����Һ��Ũ��
C����Һ��SO42������Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����480 mL���ʵ���Ũ��Ϊ0.2 mol/Lϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ________mL����Ũ����������ơ�
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________________��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��һ�����������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
�������ƹ����У�����ʵ�����ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ���_________
A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
B��ϡ���õ��ձ��Ͳ�����δϴ��
C��ϴ��������ƿδ�����������������Һ
D����Һע������ƿǰû�лָ������¾ͽ��ж���
E������ʱ���ӹ۲찼Һ��
F����ˮ�����̶��ߺ��ý�ͷ�ι����������Һ��
���ֽ�100mL��������300mL 0.4mol/LCuSO4��Һ��ϣ�����仯���Բ��ƣ�������Һ��SO42�������ʵ���Ũ����_________mol/L��

��1����Ũ��������ʵ���Ũ��Ϊ__________mol/L��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ�� ���Ķ��ٶ��仯����__________��
A����Һ��H2SO4�����ʵ��� B����Һ��Ũ��
C����Һ��SO42������Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����480 mL���ʵ���Ũ��Ϊ0.2 mol/Lϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ________mL����Ũ����������ơ�
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________________��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��һ�����������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
�������ƹ����У�����ʵ�����ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ���_________
A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
B��ϡ���õ��ձ��Ͳ�����δϴ��
C��ϴ��������ƿδ�����������������Һ
D����Һע������ƿǰû�лָ������¾ͽ��ж���
E������ʱ���ӹ۲찼Һ��
F����ˮ�����̶��ߺ��ý�ͷ�ι����������Һ��
���ֽ�100mL��������300mL 0.4mol/LCuSO4��Һ��ϣ�����仯���Բ��ƣ�������Һ��SO42�������ʵ���Ũ����_________mol/L��
19����1��18.4 ��2��BD��3����5.4 ��BCAFED ��ADE ��0.35
�����������1����c="1000�Ѧ�/M" =18.4mol/L��
��2�� ��Һ��Ũ�ȡ���Һ���ܶȲ�����ȡ��Һ��������ı䡣
��3��������Ҫ��ȡ��Һ�������Vml:����480 mL��0.2 mol/L="V��18.4" mol/L��ã�V ="5.4" mL ������˳��Ϊ�����㡢�������ܽ⡢ϴ�ӡ�ת�ơ����ݡ�ҡ�ȡ��ݴ˿�ѡ��𰸡�
��A������ȡŨ����������������ֵ��B������ʧ���ʣ�C������Ӱ�죻D����Һ��������������Һ���С������ֵ��E������ˮ�����С������ֵ��F������ʧ���ʡ�����ʹ��Һ���ʵ���Ũ��ƫ�ߵ���ADE��
��n(SO42��)="0.1L��0.2" mol/L+0.3L��0.4 mol/L="0.12" mol.����C="n/V=0.12mol/0.4L=0.35" mol/L.

��ϰ��ϵ�д�
 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
�����Ŀ
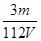 mol��L��1
mol��L��1