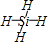��Ŀ����
��12�֣�
A.ԭ������ͬ������������ͬ�ķ��ӣ�����Ϊ�ȵ����塣
��.��֪A��B��C��D��E���ַ�������ԭ�ӵ���Ŀ����Ϊ1��2��3��6��6���Ҷ�����18�����ӣ���֪B��C��D��������Ԫ�ص�ԭ����ɣ���D����������ԭ�Ӹ�����Ϊ1 :2��
��ش�
��1�����A���ӵ�ԭ�ӵ�Ԫ�ط����� ����֪E���ж����л��E���ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ����____________________________________��
��2��C������ṹ�� ____ �Σ��÷������� ���ӣ�����ԡ��Ǽ��ԡ�����
��3������пɳ���������������D��Ϊȼ�Ϸ�Ӧ���ɵ�����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ_______ __________________ ��������Ҫд��Ӧ������
��.CO��N2��Ϊ�ȵ����塣
��4��CO���ܼ��ܴ���N2���ܼ��ܣ���CO��N2���ײμӻ�ѧ��Ӧ��
�����±����ݣ�˵��CO��N2���õ�ԭ����____________________________________��
|
|
|
A��B |
A=B |
A��B |
|
CO |
���ܣ�kJ/mol�� |
357.7 |
798.9 |
1071.9 |
|
���ܲ�ֵkJ/mol�� |
441.2 273 |
|||
|
N2 |
���ܣ�kJ/mol�� |
154.8 |
418.4 |
941.7 |
|
���ܲ�ֵkJ/mol�� |
263.6 523.3 |
��5�����ǵķ����ж�����___________���Ҽ���______________���м���
��6��Fe��Co��Ni�Ƚ�������CO��Ӧ��ԭ������Щ����ԭ�ӵĵ��Ӳ�ṹ�йء�
Niԭ�ӵļ۵����Ų�ʽΪ _____ ��Fe(CO)5�����³�Һ̬���۵�Ϊ
��20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)5�������� ____��������ͣ���Fe(CO)5������������__________ ��
B�����к��ж������������彡���ijɷ֣��ݲⶨ��Ҷ�к���450�����ϵ��л��ɷ���15�����ϵ�Ԫ�ء�ij��ѧ�о�С����̽����Ҷ�и�Ԫ�صĺ����������̽��ʵ�鷽�����£�����֪��Ҷ�е�������Ԫ�ضԸ����ӵIJⶨ��Ӱ�죩
����1����ȡ500g����IJ�Ҷ������ͨ����У��������ʹ��Ҷ�һ��������в�ĥϸ�������ձ��У�Ȼ��200mL 1 mol��L-1���������н��衢���ˡ�ϴ�ӡ�
����2������1������Һ����μ���ϡ����������Һ��������Һ��pH��6��7���ң�ʹ������Ԫ���������������ʽ��ȫ�������ټ������30 min������7.95g��ˮ̼���ƣ���ֽ��裬��������ȫ���ˣ�ϴ�ӣ����˺�õ���Һ�ͳ�����
����3��������2���õ���Һϡ����500 mL��ȡ���е�20.00 mL��Һ�Լ�����ָʾ������0.100mol��L-1��HCl����Һ�ζ����յ�ʱ������������Ϊ20.00mL����������
��ش��������⣺
���� 1�У�ʹ��Ҷ�һ�ʱ��Ҫ�õ����Ǽܡ������ǡ��ƾ���ơ� ___ �� ____ ��������
����2�У������Լ� _______ (д�Լ�����)������pH����Ϊ���㣻�жϳ����Ѿ�ϴ���ķ����� ��
����3�У��ζ������У��۾�Ӧע�� ____________________�����ζ���20 mL��Һ�к�CO32�������ʵ���Ϊ __ mol���Լ���ԭ500g��Ҷ�и����ӵ���������Ϊ _______ ������������£�
A.��1��Ar ��1�֣� E��CH3OH��CH3OH�γɷ��Ӽ������1�֣�
��2��V�Σ�����λ����������𰸣�����1�֣����Է��ӡ���1�֣�
��3��N2O4 + 2N2H4 = 3N2 + 4H2O��2�֣�
��4��CO�е�һ���м��ļ��ܱ�N2��С�ܶ࣬CO�ĵ�һ�������ϡ���1�֣�
��5��1��2��2�֣�
��6��3d84s2��1�֣������Ӿ��壨1�֣���CO��1�֣���
B������1������������ǯ��ÿ��1��,��2�֣�
����2����ˮ��2�֣�
ȡ���һ��ϴ��Һ�������Թ��У��μ�CaCl2��Һ�����������Ĵ𰸵Ⱦ��ɣ�������������������ϴ������2�֣����������𰸾����֣�
����3����ƿ����Һ��ɫ�仯�͵ζ�����Һ���������ʡ���2�֣�ֻ��ǰ��Ҳ���֣�
0.001 ��1�֣� 0.4%����0.004����1�֣�
������̣���2�֣�
����2���õ���Һ�к�CO32�������ʵ���Ϊ0.001mol��500��20=0.025mol
7.95g��ˮ̼���Ƶ����ʵ���Ϊ7.95g��106g/mol=0.075mol
ԭ500g��Ҷ�и�����(ʵ����ת��ΪCaCO3) �����ʵ���
=ת��ΪCaCO3��CO32�������ʵ���=0.075mol-0.025mol=0.050mol
ԭ500g��Ҷ�и����ӵ���������=0.050mol��40g/mol��500g=0.004
��������