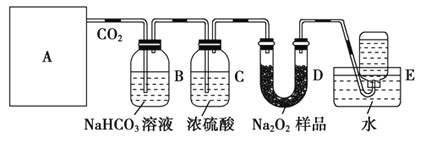
��Ŀ����
����Ŀ����һ�����·�Ӧ����ľ̿��ˮ�Ʊ�ˮú�� ������طֽ� ��ըҩ��ը ���������кͷ�Ӧ ����ʯ����ˮ��������ʯ�� �� Ba(OH)2��8H2O��NH4Cl�����ڷ��ȷ�Ӧ���� ������ţ���д����Ӧ���Ļ�ѧ����ʽ__ ��
������Ϊ�˺������û�ѧ��,ȷ����ȫ����,���������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ�,����ȡ��Ӧ��ʩ.��ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ,Ҳ�ɽ����������㡣
��1����ϩ��ʯ����ҵ����Ҫԭ��֮һ�������г��Ա�ϩ�����������������������Ʊ�ϩ�ļ��������ܵ����ӡ�ij�о�������õ�C3H8(g)===3C(ʯī��s)��4H2(g)�Ħ�H����ֱ�Ӳⶨʵ���ѽ��У���ͨ����ͼ�и������������ܺͼ����ת���е������仯����õ���

���жϣ���H________0(ѡ�>������<������)��
����H��___________(�ú���H1����H2����H3�Ĵ���ʽ��ʾ)��
��2��ʵ����,5g�״��������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ������,��д���״�ȼ�յ��Ȼ�ѧ����ʽ:_________________��
��3������̬��̬ԭ���γ�1mol��ѧ���ͷŵ���������м���.�ӻ�ѧ���ĽǶȷ���,��ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ���Ķ��Ѻ�������Ļ�ѧ�����γɹ���.�ڻ�ѧ��Ӧ������,��ѧ����Ҫ��������,�γɻ�ѧ���ֻ��ͷ�����.��֪��ӦN2(g)��3H2(g)![]() 2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݼ���a����ֵ�� (д�� + ��)��[
2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݼ���a����ֵ�� (д�� + ��)��[
��ѧ�� | H��H | N��H | N��N |
����/kJ��mol��1 | 436 | 391 | 945 |
��4�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ�ķ�Ӧ�Ƚ������㡣
��֪��C(ʯī��s)��O2(g)��CO2(g) ��H1����393.5kJ��mol��1
2H2(g)��O2(g)��2H2O(l) ��H2����571.6kJ��mol��1
2C2H2(g)��5O2(g)��4CO2(g)��2H2O(l) ��H3����2599kJ��mol��1
���ݸ�˹����������298Kʱ��C(ʯī��s)��H2(g)����C2H2(g)��Ӧ�ķ�Ӧ���Ȼ�ѧ����ʽ: _ _ _��
���𰸡���һ���ۢܢ���Ba(OH)2��8H2(OH)2O��2NH4Cl��BaCl2��10H2O��2NH3��
��������1������ �ڡ�H1����H2����H3��
��2��CH3OH(l)��3/2O2(g)=CO2(g)��2H2O(l) ��H��726.4kJ/mol
��3����93kJ/mol
��4��2C(ʯī��s)��H2(g)��C2H2(g) ��H����226.7 kJ��mol��1
��������
�����������һ�����·�Ӧ����ľ̿��ˮ�Ʊ�ˮú���������ȷ�Ӧ��������طֽ��������ȷ�Ӧ����ըҩ��ը���ڷ��ȷ�Ӧ�����������кͷ�Ӧ���ڷ��ȷ�Ӧ������ʯ����ˮ��������ʯ�����ڷ��ȷ�Ӧ���� Ba(OH)2��8H2O��NH4Cl��Ӧ�������ȷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪBa(OH)2��8H2(OH)2O��2NH4Cl��BaCl2��10H2O��2NH3����
��������1�������������仯ʾ��ͼ��֪��Ӧ�����������������������������˷�Ӧ�����ȷ�Ӧ������H��0�������������仯ʾ��ͼ��֪�������壨C3H8���ֽ�õ�ʯī��C�����������Ȼ�ѧ����ʽΪ��C3H8��g���T3C��ʯī��s��+4H2��g����H=��H1����H2����H3��
��2��ȼ������ָ��һ��������1mol��ȼ����ȫȼ�������ҵĵ�������ʱ���ų���������5g�״��������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ����������1mol�״���ȫȼ�շų���������113.5kJ��![]() ��726.4kJ����˷�Ӧ���Ȼ�ѧ����ʽΪCH3OH(l)��3/2O2(g)=CO2(g)��2H2O(l) ��H��726.4kJ/mol��
��726.4kJ����˷�Ӧ���Ȼ�ѧ����ʽΪCH3OH(l)��3/2O2(g)=CO2(g)��2H2O(l) ��H��726.4kJ/mol��
��3����Ӧ�ȵ��ڷ�Ӧ���м���֮�����������м���֮�͵IJ�ֵ����˸÷�Ӧ����Ӧ����H��945 kJ��mol��1+3��436 kJ��mol��1��2��3��391 kJ��mol��1����93kJ��mol��1��
��4������Ӧ��C(ʯī��s)��O2(g)��CO2(g) ��H1����393.5kJ��mol��1��
��Ӧ��2H2(g)��O2(g)��2H2O(l) ��H2����571.6kJ��mol��1��
��Ӧ��2C2H2(g)��5O2(g)��4CO2(g)��2H2O(l) ��H3����2599kJ��mol��1���ӣ�
������4+�������õ�4C(��ʯīs)��2H2(g)��2 C2H2(g)�����Է�Ӧ����H����453.4 kJ��mol��1������1mol C2H2(g)��Ӧ�ķ�Ӧ����H����226.7 kJ��mol��1������Ȼ�ѧ����ʽΪ2C(ʯī��s)��H2(g)��C2H2(g) ��H����226.7 kJ��mol��1��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������ж�ȫ����ȷ��һ���ǣ��� ��
A | B | C | D | |
ǿ����� | NaCl | H2SO4 | CaF2 | ʯī |
������� | HF | BaSO4 | HClO | NH3��H2O |
�ǵ���� | Cl2 | CS2 | CCl4 | ���� |
A. A B. B C. C D. D