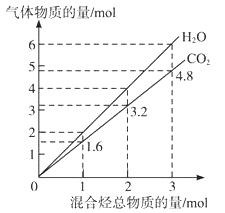
��Ŀ����
����Ŀ�������£���20 mL 0.2 mol/L H2A��Һ�еμ�0.2 mol/L NaOH��Һ���й��������ʵ����仯��ͼ������I����H2A��II����HA����III����A2������������ͼͼʾ�жϣ�����˵����ȷ����
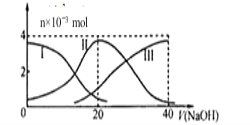
A. ��V(NaOH)=20mLʱ����Һ������Ũ�ȴ�С��ϵ��c(Na+)��c(A2��)��c(H+)��c(HA��)��c(OH��)
B. �������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�Ĵ�
C. ��V(NaOH)=30 mLʱ����Һ�д������¹�ϵ��2c(H+)+3c(H2A)+c(HA��)= c(A2��)+2c(OH��)
D. ��NaHA��Һ����ˮ�Ĺ����У�pH��������Ҳ���ܼ�С
���𰸡�C
��������
A.��V��NaOH��=20mLʱ��������ӦΪNaOH+H2A=NaHA+H2O����Һ��ҪΪNaHA��HA-�������ˮ�⣬��Һ�����ԣ���c��Na+����c��HA-����c��H+����c��A2-����c��OH-������A������
B.�������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����ͼʾ��ϵ֪��c��A2-����c��H2A����˵���������ˮ��̶ȣ���Һ�����ԣ���Һ��ˮ�ĵ����ܵ������ƣ��ȴ�ˮС����B����
C.��V��NaOH��=30mLʱ��������ӦΪNaOH+H2A=NaHA+H2O��NaHA+NaOH=Na2A+H2O����Һ��ҪΪ����������NaHA��Na2A�Ļ����Һ�����ݵ���غ�ã�c��Na+��+c��H+��=c��HA-��+2c��A2-��+c��OH-���٣������غ��֪��3c��HA-��+3c��A2-��+3c��H2A��=2c��Na+����������2+�ڵã�2c��H+��+c��HA-��+3c��H2A���Tc��A2-��+2c��OH-������C��ȷ��
D.������V��NaOH��=20mLʱ����ʱ����ΪNaHA������ͼ�����֪������Һ��c��A2-����c��H2A����˵��HA-�ĵ���̶ȴ���ˮ��̶�����Һ��ʾ���ԣ���D������
��ѡC��

 ��У����ϵ�д�
��У����ϵ�д�