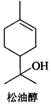
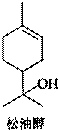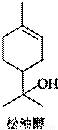��Ŀ����
��5�֣����ʹ���һ����Ȼ�л�����������ڷ����͡������ͼ��Ȼ��͵���Ȼֲ�ᆱ���У������϶��������������Ϲ�ҵ����;�㷺����Ҫ������������ױƷ���Ϻ͵����㾫��

8-1��д�����ʹ��ֱ��������Լ���Ӧ������Ҫ�л�����Ľṹʽ�������������칹�壩��
��1��Br2/CC14����2��KMnO4��ϡ������Һ������3��(a) KMnO4/NaOH��(b) H3O+����4��PBr3����5��(a) Na��(b) CH3I����6��CH3COC1/�
8-2���ʹ��Ƿ���������칹�壨��ӳ�칹�壩�����У���ָ���÷�����������̼ԭ�ӵ���Ŀ�����ܴ��ڵ������칹�����Ŀ��
8-1 ��С��3�֣�ÿ��0.5��
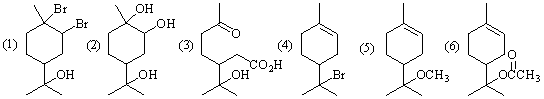
8-2����2��
���������칹�壨��𣺴��ڶ�ӳ�칹�壬����ǣ�����
����������̼ԭ�ӡ�0.5�֣������ܴ��ڣ��������칹�塡0.5��

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ
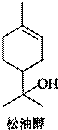 ���ʹ���һ����Ȼ�л�����������ڷ����͡������ͼ��Ȼ��͵���Ȼֲ�ᆱ���У������϶��������������Ϲ�ҵ����;�㷺����Ҫ������������ױƷ���Ϻ͵����㾫�����ʹ��ṹ��ͼ��ʾ�������й����ʹ���������ȷ���ǣ�������
���ʹ���һ����Ȼ�л�����������ڷ����͡������ͼ��Ȼ��͵���Ȼֲ�ᆱ���У������϶��������������Ϲ�ҵ����;�㷺����Ҫ������������ױƷ���Ϻ͵����㾫�����ʹ��ṹ��ͼ��ʾ�������й����ʹ���������ȷ���ǣ�������