��Ŀ����
����Ŀ����ѧ�Լ�Ӧ���������ʺ���̬������Ч���Ʊ��棺
(1)�����Լ�Ӧ������____________�У�Һ���Լ�������____________�У������ֽ���Լ�(�磺ŨHNO3��AgNO3��)Ӧ��____________ɫ�Լ�ƿ���档�����(HF)��Ͳ����е�SiO2ǿ�ҷ�Ӧ����ʴ������Ӧ��____________��ţ������ơ���Ӧ������____________�У���������Ӧ�ñ�����____________�С�
(2)������ˮ�豣������ɫ�Լ�ƿ�У�ԭ����__________________��
(3)ʵ���������Ƶ�����������Һ��һ��Ҫ��������___________����ֹ�ڿ����б��ʡ�
(4)��ʢ��Ũ������Լ�ƿ��ǩ��ӡ�����о�ʾ��ǣ�˵��Ũ�������_______�ԡ�

���𰸡����ƿ ϸ��ƿ �� ����ƿ ú�� ��ˮ ������ˮ�к��д����ᣬ����ֽ������ ����(����м) ��ʴ
��������
(1)�������ڹ��ƿ�У�Һ���Լ�������ϸ��ƿ�У������ֽ��������ܹⱣ�棬Ӧ����ɫ�Լ�ƿ���棻����ᣨHF����Ͳ����е�SiO2ǿ�ҷ�Ӧ�������ò���ƿ��š���������ƿ��ţ������ơ�������ˮ�������ȷ�Ӧ��Ӧ������ú���У������ڿ���������ȼ����������Ӧ�ñ�������ˮ�У�
���ǣ����ƿ��ϸ��ƿ���أ�����ƿ��ú�ͣ���ˮ��
(2) ������ˮ�к��д����ᣬ������ȶ�������ֽ⣬���豣������ɫ�Լ�ƿ�У�
���ǣ�������ˮ�к��д����ᣬ����ֽ�����ʣ�
(3)�������Ӿ���ǿ�Ļ�ԭ�ԣ��ڿ������ױ���������Ϊ�����ӣ���˿����������Ƶ�����������Һ�м������������ۣ���ֹ�������ӱ�����Ϊ�����ӣ��ڿ����б��ʣ�
���ǣ�����(����м)��
(4) ��ʾ���Ϊ��ʴ��Һ��ı�־��˵��Ũ������и�ʴ�ԣ�
�ʴ��ǣ���ʴ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������������������Ϸ�Ӧ������NH4Cl(�׳���ɰ)��һ������Ⱦ�����塣ijѧϰС����������װ��ģ��÷�Ӧ,��ش�������⡣

(1)��ʵ�����������������Ũ������MnO2��Ӧ��ȡ��װ��A������X������Ϊ____;X�з�����Ӧ�����ӷ���ʽΪ_______��
(2)Ҫ�õ����﴿��������,����A��B��Cװ�õ�����˳������Ϊa��_____(��Сд��ĸ��ʾ)��
(3)����Eװ��,������������������ֻ�Ϸ�Ӧ����Ӧ��_______(��Сд��ĸ��ʾ)ͨ��,��Ӧ�Ļ�ѧ����ʽΪ_______________;���ܹ۲쵽��������___________��
(4)���Ʊ��ݡ�������ɰ��ҩ����ɢ������,��Ȼ��ɰ������NH4Cl����ȡ��Ȼ��ɰ����NH4Cl�����ⶨ��ȷ��ȡһ��������ɰ,������������ͭ���,����ͼ��ʾ����ʵ�顣��֪:2NH4Cl+3CuO=3Cu+N2+2HCl+3H2O ��
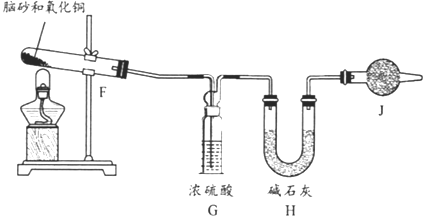
��Ϊ˳������й��������IJⶨ,���������б���:
����ʱ��� | ����ǰ | ���۲쵽_____����ʱ |
�������� | _______________ | ֹͣ����,��ȴ,����Hװ�õ������� |
���������Jװ��,���NH4Cl�ĺ�����_____(����ƫ������ƫ����������Ӱ����)��
����Ŀ�����Ȼ�����(S2Cl2)�ڹ�ҵ����������Ϊ��ʵ���Һϳ�S2Cl2��ij��ѧ�о���ѧϰС��������й����ϣ��õ�������Ϣ��
�ٽ������������110�桫140������Ӧ�����ɵ�S2Cl2��Ʒ��
���й����ʵIJ����������±���
���� | �۵�/�� | �е�/�� | ��ѧ���� |
S | 112.8 | 444.6 | �� |
S2Cl2 | ��77 | 137 | ��ˮ����HCl��SO2��S���������������S2Cl2��Cl2 �¶ȳ�300��������ȫ�ֽ�Ϊ������� |
���ʵ��װ��ͼ���£�

(1)��ͼ�����巢����β������װ�ò������ƣ���������Ľ����_______________________��
���øĽ������ȷװ�ý���ʵ�飬��ش��������⣺
(2)B�з�Ӧ�����ӷ���ʽΪ_________________________________________________________��
(3)C��D�е��Լ��ֱ���_________________��_____________________��
(4)����A��B�����Ʒֱ���___________��___________��F��������_______________________��
(5)����ڼ���Eʱ�¶ȹ��ߣ���ʵ������Ӱ��_________________________________����F�п��ܳ��ֵ�������________________________________________________��
(6)S2Cl2��Ʒ�п��ܻ��е�������(��д�������ʵĻ�ѧʽ)______________��_____________��Ϊ�����S2Cl2�Ĵ��ȣ��ؼ��IJ����ǿ��ƺ��¶Ⱥ�_____________________________________��