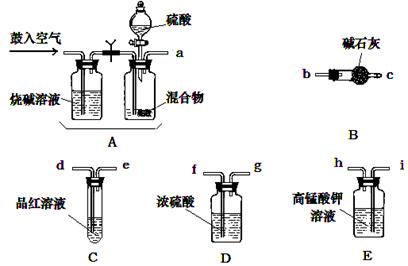
��Ŀ����
��NH4HCO3��NH4Cl��Na2CO3��xH2O�Ļ���ﹲ11.62g������44mL5mol��L-1������NaOH��Һ���ȳ�ַ�Ӧ��ʹ�ų�������ͨ����ʯ�ң��ռ�����״���µ�����3.36L��������Һ�м���30mL2.5mol��L-1H2SO4��Һ�����Լ���ʹ������ȫ�ų����ռ�����״���¸��������1.344L���ѷ�Ӧ�����Һϡ�͵�100mL�����H+��Ũ��Ϊ0.1mol��L-1������1��ԭ������и����ʵ�����
��2��Na2CO3��xH2O�е�xֵ��
����ʾ����ο�����NaOH��Һ��Ӧ���ɰ�����
�𰸣�
������
������
| �⣺������H+��0.1mol/L´0.1L=0.01mol
H2SO4��Na2CO3��Ӧ����CO2Ϊ1.344L��0.06mol����ʱ����H2SO40.06mol�к�NaOH�����ĵ�H2SO4Ϊ�� 0.03L´2.5mol��L- �μӷ�Ӧ��NaOH�� 0.044L´5mol��L-1-2´0.01mol=0.2mol ����NH33.36L����0.15mol ������NaOH0.15mol�к�HCO3-��NaOHΪ0.05mol ��NH4HCO3Ϊ0.05mol����3.95g��NH4ClΪ0.1mol����5.35g��Na2CO3��xH2OΪ2.32g ��n(CO2)=0.06mol n(NH4HCO3)=0.05mol ��Na2CO3��cH2OΪ0.01mol 0.01mol´(106+18x)g��mol-1=2.32g ��c=7 ����
|

��ϰ��ϵ�д�
�����Ŀ