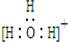��Ŀ����
��������(H2O2)��һ����ɫ��Һ��,����ˮ��Һ�׳�˫��ˮ,��������,����������������ɱ������Ư���ȡ�
(1)����˵����ȷ���� ��
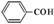
(2)H2O2��һ�ֶ�Ԫ����,��д�����ĵڶ������뷽��ʽ: ��
(3)��H2O2��Һ��������FeCl2��Һ��,��Һ��dz��ɫ��Ϊ�ػ�ɫ,д���÷�Ӧ�����ӷ���ʽ: ��
(4)ij����ҵ��ˮ�к���һ��������,Ϊ�˳�ȥ����,������H2O2�����ȼ�,д���÷�Ӧ�Ļ�ѧ����ʽ: ��
(1)����˵����ȷ���� ��
| A��������������м��м��Լ����зǼ��Լ� |
| B��H2O2��H2O��Ϊͬ�������� |
| C��34 g H2O2�к��е���������ΪNA |
| D��ʵ���ҿ������ù���������ȡ���� |
(3)��H2O2��Һ��������FeCl2��Һ��,��Һ��dz��ɫ��Ϊ�ػ�ɫ,д���÷�Ӧ�����ӷ���ʽ: ��
(4)ij����ҵ��ˮ�к���һ��������,Ϊ�˳�ȥ����,������H2O2�����ȼ�,д���÷�Ӧ�Ļ�ѧ����ʽ: ��
(1)AD
(2)H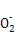

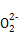 +H+
+H+
(3)H2O2+2Fe2++2H+=2Fe3++2H2O
(4)H2O2+Cl2=2HCl+O2��
(2)H
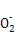

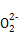 +H+
+H+(3)H2O2+2Fe2++2H+=2Fe3++2H2O
(4)H2O2+Cl2=2HCl+O2��
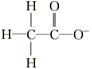
(1)��ͬ��Ԫ���γɵIJ�ͬ���ʻ�Ϊͬ��������,B��;H2O2���ڹ��ۻ�����,����������,C����(2)����ֲ�����:H2O2 H
H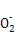 +H+��H
+H+��H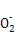

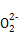 +H+��(3)H2O2����ǿ������,���������½�FeCl2����ΪFeCl3,��������ԭΪH2O:H2O2+2Fe2++2H+=2Fe3++2H2O��(4)H2O2�����ȼ�ʱ,H2O2����ԭ��,��Cl2��ԭΪHCl,����������ΪO2:H2O2+Cl2=2HCl+O2��
+H+��(3)H2O2����ǿ������,���������½�FeCl2����ΪFeCl3,��������ԭΪH2O:H2O2+2Fe2++2H+=2Fe3++2H2O��(4)H2O2�����ȼ�ʱ,H2O2����ԭ��,��Cl2��ԭΪHCl,����������ΪO2:H2O2+Cl2=2HCl+O2��
 H
H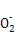 +H+��H
+H+��H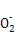

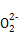 +H+��(3)H2O2����ǿ������,���������½�FeCl2����ΪFeCl3,��������ԭΪH2O:H2O2+2Fe2++2H+=2Fe3++2H2O��(4)H2O2�����ȼ�ʱ,H2O2����ԭ��,��Cl2��ԭΪHCl,����������ΪO2:H2O2+Cl2=2HCl+O2��
+H+��(3)H2O2����ǿ������,���������½�FeCl2����ΪFeCl3,��������ԭΪH2O:H2O2+2Fe2++2H+=2Fe3++2H2O��(4)H2O2�����ȼ�ʱ,H2O2����ԭ��,��Cl2��ԭΪHCl,����������ΪO2:H2O2+Cl2=2HCl+O2��
��ϰ��ϵ�д�
�����Ŀ
 ��ʾ14Cԭ�ӽṹʾ��ͼ
��ʾ14Cԭ�ӽṹʾ��ͼ ��ʾCCl4���ӵı���ģ��
��ʾCCl4���ӵı���ģ��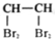 ��ʾ1��1��2��2-��������
��ʾ1��1��2��2-��������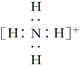
 Ce��
Ce�� Ce��
Ce�� Ce��
Ce��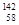 Ce�����ǻ���Ϊͬ��������
Ce�����ǻ���Ϊͬ��������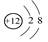
 Cl
Cl