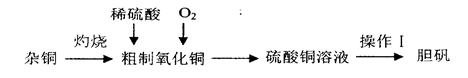
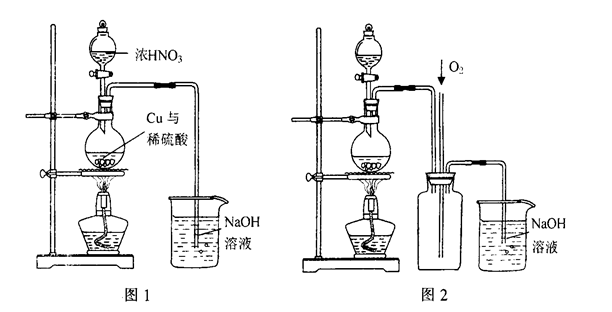
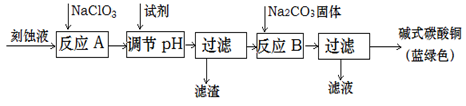
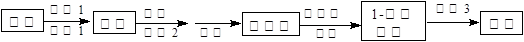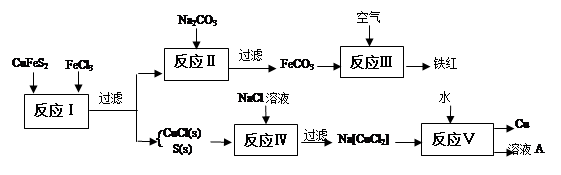��Ŀ����
���������(K2S2O8)��һ����ɫ�ᾧ���������Ҵ�����ǿ�����ԣ��ֽ⡣ʵ�����Ʊ���������ؿ�ͨ�����µ��KHSO4����Һ�õ���
ʵ�鲽�����£�
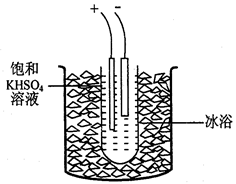
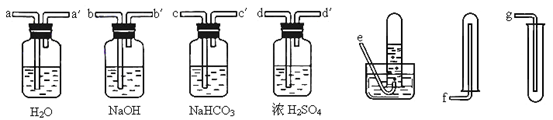
����1����ȡ40gKHSO4�ܽ�90mL����ˮ��������Թܣ��Թܽ��ڱ�ˮԡ��(װ�ü�ͼ9)������ȴ��5�����¡�
����2���2h��ÿ��Լ��Сʱ��һ�α�
����3���������ռ���©���У�ֱ�����Ҵ�������ϴ�Ӻ���
����4���������
����5���������Ѻ��Ҵ�
��1������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��2���������У���������������ʹʪ���KI-���۱�������ɫ�������壬�����������(�ѧʽ)��
��3������2ÿ����СʱҪ����ձ����ӱ��飬��ԭ���� ��
��4������5�����Ҵ�������ʱ���õIJ��������� ��
��5��ȡ�õ�����Ʒ0.2500g����30mLˮ����4gKI����סƿ��������ֹ15min������1mL�����ᣬ����cmol��L- 1Na2S2O3��Һ�ζ���(S2O82- +3I- =2SO42- +I3-��I3- I2+I-��2S2O32-+I2=2I- + S4O62-)
I2+I-��2S2O32-+I2=2I- + S4O62-)
���ܽ�ʱ������KI������סƿ������Ŀ���� ��
�ڱ�ʵ�����õ�ָʾ��Ϊ ��
�������εζ�����Na2SO3��ҺVmL���ɱ��ν�����㣬��Ʒ��K2S2O8�Ĵ���Ϊ(�ú�c��V�Ĵ���ʽ��ʾ)��
��6��������ѧ�ϼ���Mn2+��Ag+����K2S2O8��Һ��Mn2+����Ϊ��ɫ��MnO4-���÷�Ӧ�����ӷ���ʽΪ ��
ʵ�鲽�����£�
����1����ȡ40gKHSO4�ܽ�90mL����ˮ��������Թܣ��Թܽ��ڱ�ˮԡ��(װ�ü�ͼ9)������ȴ��5�����¡�

����2���2h��ÿ��Լ��Сʱ��һ�α�
����3���������ռ���©���У�ֱ�����Ҵ�������ϴ�Ӻ���
����4���������
����5���������Ѻ��Ҵ�
��1������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��2���������У���������������ʹʪ���KI-���۱�������ɫ�������壬�����������(�ѧʽ)��
��3������2ÿ����СʱҪ����ձ����ӱ��飬��ԭ���� ��
��4������5�����Ҵ�������ʱ���õIJ��������� ��
��5��ȡ�õ�����Ʒ0.2500g����30mLˮ����4gKI����סƿ��������ֹ15min������1mL�����ᣬ����cmol��L- 1Na2S2O3��Һ�ζ���(S2O82- +3I- =2SO42- +I3-��I3-
 I2+I-��2S2O32-+I2=2I- + S4O62-)
I2+I-��2S2O32-+I2=2I- + S4O62-)���ܽ�ʱ������KI������סƿ������Ŀ���� ��
�ڱ�ʵ�����õ�ָʾ��Ϊ ��
�������εζ�����Na2SO3��ҺVmL���ɱ��ν�����㣬��Ʒ��K2S2O8�Ĵ���Ϊ(�ú�c��V�Ĵ���ʽ��ʾ)��
��6��������ѧ�ϼ���Mn2+��Ag+����K2S2O8��Һ��Mn2+����Ϊ��ɫ��MnO4-���÷�Ӧ�����ӷ���ʽΪ ��
��1��2KHSO4
 K2S2O8 + H2��
K2S2O8 + H2����2��O3
��3������ˮ�£���֤���������¶���5������
��4������
��5���ٷ�ֹI�������������ƫ������ �ڵ�����Һ��54cV%
��6��2Mn2++5S2O82�� +8H2O
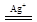 2MnO4�� +10SO42�� +16H+
2MnO4�� +10SO42�� +16H+�����������1�����KHSO4��Һ��SO42��ʧ��������S2O82-��H���õ�������H2����2������Ϊ�����ӷŵ磬����������I�������壬��Ϊ��ɫ����ӦΪO3���������OH���ŵ�����O2��O2�ڷŵ������½�һ��ת��ΪO3����3������Ϣ֪������������ֽ⣬����Ҫ�ڵ��������ɡ���4���Ҵ�������Ϊ��ܽ���л�����������߷е㲻ͬ������ֿ�����5��I���ױ�������������е�O2����ʵ������Ҫ���ܡ�������I2����ɫ����ʵ���е�I2������ʱ����ɫ����ȥ����ָʾ��Ӧ���յ㡣�ɷ���ʽ�ҳ���ϵʽ��
K2S2O8��I3�� ��I2��2S2O32��
270 2
m(K2S2O8) cV10-3
w(K2S2O8)= m(K2S2O8)/0.25 =54cv%��
��6��Mn��+2������+7�ۣ�S��+7����+6�����ݵ�ʧ�����غ���ƽ��Ӧ�����ɵ���غ�ȷ������������H���������Hԭ���غ㣬ȷ����Ӧ���л���H2O������ƽ��

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
�����Ŀ
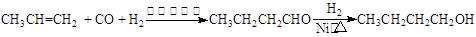
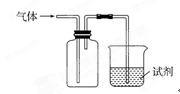
 CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã�����ͼ��
CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã�����ͼ��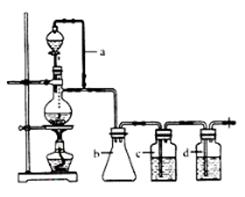
 RCH(OH)SO3Na�����ڷе㣺����34�棬
RCH(OH)SO3Na�����ڷе㣺����34�棬