��Ŀ����
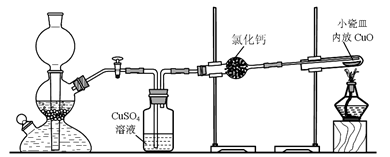
��ʽ̼��ͭ��һ����;�㷺�Ļ���ԭ�ϡ���ҵ�Ͽ������Կ�ʴ��Һ����Ҫ����Cu2+��Fe2+��Fe3+��H +��Cl-���Ʊ������Ʊ��������£�
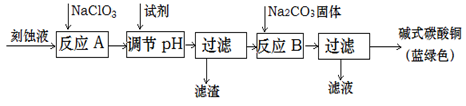
Cu2+��Fe2+��Fe3+���ɳ�����pH���£�
��1����������Ҫ�ɷ��� ��д��ѧʽ����
��2�����ڷ�ӦA����Һ��pH��ΧӦΪ ��ѡ����Լ�����ʵ��� ������ţ���
a����ˮ b��ϡ���� c���������� d��̼��ͭ
��3����ӦB���¶�����ߣ�����������ɫ��Ʒ�п��ܻ���ֵ������� ��
��4����Na2CO3��Һ���뵽һ����CuCl2��Һ�еõ�����������Ӧ�����ӷ���ʽ��ʾ��
�� ����ֻ��CuCO3�� ��
�� ����ֻ��Cu(OH)2�ҷų����ݣ� ��
��5����ʽ̼��ͭ����ɿɱ�ʾΪ��aCuCO3?bCu(OH)2?cH2O��Ҫ�ⶨ����ɣ���ͬѧ��Ƶ�ʵ�鷽������Ҫ����������裺�ٳ�����Ʒ���������ڸ��·ֽ⣻�۲��CO2���������ܲ��ˮ�������������ݳ���CuO������������ͬѧ��Ϊ��������⣬ʵ��ֻ��ⶨ�ĸ����е��������ɣ�����������Ϊ ������ţ�дһ�鼴�ɣ���

Cu2+��Fe2+��Fe3+���ɳ�����pH���£�
| �� �� | Cu(OH)2 | Fe (OH)2 | Fe (OH)3 |
| ��ʼ����pH | 4.2 | 5.8 | 1.2 |
| ��ȫ����pH | 6.7 | 8.3 | 3.2 |
��1����������Ҫ�ɷ��� ��д��ѧʽ����
��2�����ڷ�ӦA����Һ��pH��ΧӦΪ ��ѡ����Լ�����ʵ��� ������ţ���
a����ˮ b��ϡ���� c���������� d��̼��ͭ
��3����ӦB���¶�����ߣ�����������ɫ��Ʒ�п��ܻ���ֵ������� ��
��4����Na2CO3��Һ���뵽һ����CuCl2��Һ�еõ�����������Ӧ�����ӷ���ʽ��ʾ��
�� ����ֻ��CuCO3�� ��
�� ����ֻ��Cu(OH)2�ҷų����ݣ� ��
��5����ʽ̼��ͭ����ɿɱ�ʾΪ��aCuCO3?bCu(OH)2?cH2O��Ҫ�ⶨ����ɣ���ͬѧ��Ƶ�ʵ�鷽������Ҫ����������裺�ٳ�����Ʒ���������ڸ��·ֽ⣻�۲��CO2���������ܲ��ˮ�������������ݳ���CuO������������ͬѧ��Ϊ��������⣬ʵ��ֻ��ⶨ�ĸ����е��������ɣ�����������Ϊ ������ţ�дһ�鼴�ɣ���
��15�֣���ע����ÿ��2�֣�
��1��Fe(OH)3 �� ��2��3.2��4.2�� d�� ��3��CuO��������ɣ���
��4����Cu2++CO32��=CuCO3������Cu2++CO32��+H2O= Cu(OH)2��+ CO2����
��5���٢ۢܣ���٢ۢݡ��٢ܢݣ���3�֣�
��1��Fe(OH)3 �� ��2��3.2��4.2�� d�� ��3��CuO��������ɣ���
��4����Cu2++CO32��=CuCO3������Cu2++CO32��+H2O= Cu(OH)2��+ CO2����
��5���٢ۢܣ���٢ۢݡ��٢ܢݣ���3�֣�
�����������1���Ա�ԭ�Ϻ�Ŀ�����ijɷ֣����Բ²�����̸����������ҪĿ�ģ���ӦA��Ϊ�������������ӣ�����pH��Ϊ��ʹ������ȫ����Ϊ��������������������Ϊ�˳�ȥ��Ԫ�أ������������Ҫ�ɷ���Fe(OH)3����ӦB����ȡ��ʽ̼��ͭ���ڶ��ι�����Ϊ�˳�ȥ�������Σ���2����������Ϣ������������ԭ�Ϻ�Ŀ��������ɣ�������ҺpHʱ��Ӧʹ��������ȫ��������ͭ���Ӳ��ܿ�ʼ�����������ҺpHӦ����3.2��4.2֮�䣻��ѡ��ˮ��������笠����ӵ����ʣ���aѡ�������ѡ��ϡ���ᣬ��������������ӵ����ʣ���bѡ�������ѡ���������ƣ������������ӵ����ʣ���cѡ�������ѡ��̼��ͭ�������ܳ�ȥ���ʣ����������ͭ����������Ŀ������������dѡ����ȷ����3������ӦB���¶ȹ��ߣ������ļ�ʽ̼��ͭ���ֽܷ����ɺ�ɫ������ͭ���壬ʹ�Ʊ���Ŀ����ﲻ������4��̼���������ͭ���ӽ������̼��ͭ��������Cu2++CO32��=CuCO3����ͭ������̼������ӷ���˫ˮ�ⷴӦ������������ͭ�����Ͷ�����̼���壬��Cu2++CO32��+H2O= Cu(OH)2��+ CO2������5����ʽ̼��ͭ���·ֽ��ԭ��Ϊ��aCuCO3?bCu(OH)2?cH2O
 (a+b)CuO+(b+c)H2O��+aCO2�������ڸ����ʵ�Ħ��������ֵ�ϵ���ʽ����n=m/M���÷�Ӧ�м�ʽ̼��ͭ������ͭ��ˮ������������̼��ϵ��֮�ȵ������ʵ���֮�ȣ�ֻ��Ҫ���Т٢ۢܣ���٢ۢݡ��٢ܢݣ�ʵ�飬�Ϳ��Լ������ʽ̼��ͭ�������a��b��c��ֵ��
(a+b)CuO+(b+c)H2O��+aCO2�������ڸ����ʵ�Ħ��������ֵ�ϵ���ʽ����n=m/M���÷�Ӧ�м�ʽ̼��ͭ������ͭ��ˮ������������̼��ϵ��֮�ȵ������ʵ���֮�ȣ�ֻ��Ҫ���Т٢ۢܣ���٢ۢݡ��٢ܢݣ�ʵ�飬�Ϳ��Լ������ʽ̼��ͭ�������a��b��c��ֵ��
��ϰ��ϵ�д�
�����Ŀ

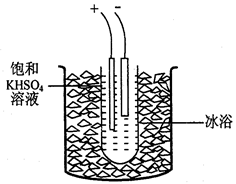
 I2+I-��2S2O32-+I2=2I- + S4O62-)
I2+I-��2S2O32-+I2=2I- + S4O62-)