��Ŀ����
����Ŀ����֪���� 2C(s)+O2(g)=2CO(g)����H= -220kJ��mol-1
�� ����ȼ�յ������仯ʾ��ͼ��
����˵����ȷ����
A.1mol C(s)��ȫȼ�շų�110 kJ������
B.H2(g)+1/2O2(g)=H2O(g)����H= -480kJ��mol-1
C.C(s)+H2O(g)=CO(g)+H2(g)����H= +130kJ��mol-1
D.���ֽ�2mol H2O(l)��������Ҫ�ṩ4��462kJ������
���𰸡�C
��������
A��C(s)��ȫȼ�յIJ���Ϊ������̼���壬1mol C(s)��ȫȼ�շų�����������110 kJ��A����
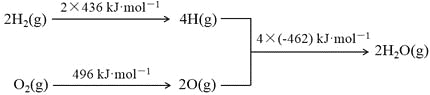
B��H2(g)+1/2O2(g)=H2O(g) ����H��![]() ��(2��436+496-4��462)= -240kJ��mol-1��B����
��(2��436+496-4��462)= -240kJ��mol-1��B����
C���� 2C(s)+O2(g)=2CO(g) ����H= -220kJ��mol-1����H2(g)+1/2O2(g)=H2O(g)�� ��H= -240kJ��mol-1�����١�![]() -�����ã�C(s)+H2O(g)=CO(g)+H2(g) ����H= +130kJ��mol-1��C��ȷ��
-�����ã�C(s)+H2O(g)=CO(g)+H2(g) ����H= +130kJ��mol-1��C��ȷ��
D������H2(g)+1/2O2(g)=H2O(g)����H= -240 kJ��mol-1�����ֽ�2mol H2O(l)��������Ҫ�ṩ2��240 kJ��������D����
��ѡC��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ����Ԫ���ں����е�ѭ����������������̬ϵͳ�Ļ����ؼ���������������ѭ�����̿�����ͼ��ʾ��
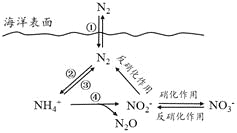
��1�������еĵ�ѭ����ʼ�ڵ��Ĺ̶����������ڹ̵����õ�һ����_______����ͼ��������ţ���
��2�����й��ں���ѭ����˵����ȷ����__________������ĸ��ţ���
a.�����д�������̬�ĵ�
b.�����еĵ�ѭ����ʼ�ڵ�������
c.�����еķ���������һ���������IJ���
d.�����ŷź�NO3-�ķ�ˮ��Ӱ�캣����NH4+�ĺ���
��3������ʱ��������ϸ�������£�NH4+��ʵ�ֹ��̢ܵ�ת���������̢ܵ����ӷ���ʽ����������
________NH4++ 5O2=2NO2-+ ________H++__________+__________
��4�������о����¶ȶԺ�������ϸ��ȥ������Ч����Ӱ�죬�±�Ϊ��10 L�˹���ˮ�����ļ�����ݣ�
�¶�/�� | ������������/mg | ����24 h | ����48 h |
��������/mg | ��������/mg | ||
20 | 1008 | 838 | 788 |
25 | 1008 | 757 | 468 |
30 | 1008 | 798 | 600 |
40 | 1008 | 977 | 910 |
����ϸ��ȥ����������ѷ�Ӧ�¶���_____________������ѷ�Ӧ�¶�ʱ��48 h��ȥ��������Ӧ��ƽ��������____________mg��L-1��h-1��
��5��Ϊ�˱��⺬����ˮ�Ժ���ѭ��ϵͳ��Ӱ�죬�辭�������ŷš���ͼ�Ǽ��������ҵ��ˮ�а�����NH4+����ʾ��ͼ��
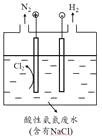
�� ��ϵ缫��Ӧʽ�������������ȥ��������ԭ����_______________��
�� ������H2��N2�����ʵ���֮��Ϊ3:1���������ˮ��pH��___________����������������������������С��������������ɣ�_______________��