��Ŀ����
ʵ�����Ʊ�������(C2H5Br)��װ�úͲ�����ͼ��(��֪������ķе�38.4��)
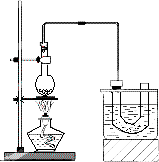
�ټ��װ�õ������ԣ���װ��ͼ��ʾ��U�ιܺʹ��ձ��м����ˮ��
����Բ����ƿ�м���10 mL��95���Ҵ���28 mL��80��Ũ���ᣬȻ�������ϸ��13 g�廯�ƺͼ������Ƭ��
��С�ļ��ȣ�ʹ���ַ�Ӧ��
�ش��������⣺
(1)��ʵ����ȡ������Ļ�ѧ����ʽΪ��________��
(2)��Ӧʱ���¶ȹ��ߣ��ɿ����к���ɫ������������������ʽΪ________��ͬʱ��������һ����ɫ���壮
(3)U���ڿɹ۲쵽��������________��
(4)��Ӧ������U�ι��д��Ƶ�C2H5Br���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ���ѡ�������Լ��е�________(����ĸ)
A��NaOH��Һ
B��H2O
C��Na2SO3��Һ
D��CCl4
�������Ҫ����������________(����������)��Ҫ��һ���Ƶô�����C2H5Br������ˮϴ��Ȼ�������ˮCaCl2���ٽ���________(���������)��
(5)���м���ʵ�鲽�裬�����ڼ�������������Ԫ�أ�����ȷ�IJ���˳���ǣ�ȡ���������飬Ȼ��________(�����)��
�ټ��ȣ�
�ڼ���AgNO3��Һ��
�ۼ���ϡHNO3�ữ��
�ܼ���NaOH��Һ��
����ȴ
(6)
������(C2H5Br)��һ��̼��ͬϵ�����п��ܵ��������������ƵĴ���Һ���ܷ�����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ________��_________��������
|
����(1)NaBr��H2SO4��C2H5OH ����(2)Br2(1��) ����(3)����״Һ������(2��) ����(4)c(1��)����Һ©��(1��)������(1��)�� ����(5)�ܢ٢ݢۢ�(2��) ����(6)CH3CH2CH2Br��NaOH ����CH3CHBrCH3��NaOH |

 ��������һ����Ҫ���л�����ԭ�ϣ���е�Ϊ38.4�森�Ʊ��������һ�ַ������Ҵ��������ᷴӦ���÷�Ӧ�Ļ�ѧ����ʽ��
��������һ����Ҫ���л�����ԭ�ϣ���е�Ϊ38.4�森�Ʊ��������һ�ַ������Ҵ��������ᷴӦ���÷�Ӧ�Ļ�ѧ����ʽ�� ��������������ˮ���ռ�����ã����ܷ����ĸ���Ӧ��H2SO4��Ũ��+2HBr��Br2+SO2+2H2O
��������������ˮ���ռ�����ã����ܷ����ĸ���Ӧ��H2SO4��Ũ��+2HBr��Br2+SO2+2H2O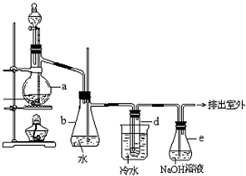 ������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������װ����ͼ��ʾ���Թ�d��װ����������ˮ����֪������ķе�Ϊ38.4��C���ܶ�Ϊ1.43g?ml-1��
������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������װ����ͼ��ʾ���Թ�d��װ����������ˮ����֪������ķе�Ϊ38.4��C���ܶ�Ϊ1.43g?ml-1��