��Ŀ����
����Ŀ������˵����ȷ���ǣ� ��
A. ��ѧƽ���������ƶ�ʱ��ƽ�ⳣ��Kֵһ������
B. HS���ĵ��뷽��ʽ��HS��+H2O![]() S2��+H3O+
S2��+H3O+
C. ��ˮ�������c(H+)��1��10��13mol/L����Һ�У����ܴ�����������ӣ�Fe3+��K+��NH4+�� SO42����Cl����ClO��
D. AlCl3��Һ��Na2CO3��Һ��Ϸ�����Ӧ��2Al3++3CO32����Al2(CO3)3��
���𰸡�B
��������A.ƽ�ⳣ��KΪ�¶ȵĺ�����ֻҪ�¶ȸı䣬ƽ��Żᷢ���ƶ���A������B.HS��Ϊ��������ӣ����ַ������룬 B��ȷ��C. ��ˮ�������c(H+)��1��10��13mol/L����Һ������Һ��Ϊ���ԡ���Ϊ���ԣ����Ի����У�ClO����H+��Ӧ���ɴ����ᣬ���Ի����У�Fe3+��OH-������������������C������D. Al3+ˮ�������ԣ�CO32��ˮ���Լ��ԣ���Ϻ���ٽ�ˮ�⣬�����������������Ͷ�����̼���壬D��������ѡB��

����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ��
����Cr(��)�Ĵ��������������£�

��֪���������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
�������£�����������������������ʽ����ʱ��Һ��pH���£�
������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
������ȫʱ��pH | 3.7 | 11.1 | 5.4(>8�ܽ�) | 9(>9�ܽ�) |
(1)ʵ������18.4 mol��L-1��Ũ��������480 mL 2 mol��L��1�����ᣬ����ȡŨ����___mL������ʱ���ò�����������Ͳ���ձ��Ͳ������⣬����____________________________��
(2)H2O2�������ǽ���Һ���е�Cr3+ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ��
___________________________________________��
(3)����NaOH��Һʹ��Һ�ʼ��ԣ��ȿ��Գ�ȥijЩ�������ӣ�ͬʱ�ֿ��Խ�Cr2O72-ת��Ϊ__________(�����Ļ�ѧʽ)
(4)�����ӽ�����֬�ķ�Ӧԭ��Ϊ��Mn+ + n NaR = MRn + n Na���������������ӽ�����֬�ɳ�ȥ��Һ���еĽ�����������__________________��
(5)д��������������SO2���л�ԭʱ������Ӧ�����ӷ���ʽ______________________________��
(6)�����ζ����Dzⶨ����Ũ�ȵķ���֮һ��Ϊ�˲ⶨij��ˮ��SCN��Ũ�ȣ����ñ�AgNO3��Һ�ζ�����Һ����֪��
�������� | AgCl | AgI | AgCN | Ag2CrO4 | AgSCN |
��ɫ | �� | �� | �� | ש�� | �� |
Ksp | 1.8��10-10 | 8.3��10-17 | 1.2��10-16 | 3.5��10-11 | 1.0��10-12 |
�ζ�ʱ��ѡΪ�ζ�ָʾ������____(ѡ����)���ζ��յ�������________________________��
A��NaCl B��K2CrO4 C��KI D��NaCN
����Ŀ����˾ƥ�֣�����ˮ������![]() ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128����135����ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ��������
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128����135����ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ��������

�Ʊ����������������£�
![]()
��Ҫ�Լ��Ͳ�Ʒ�������������±���ʾ��
���� | ��Է������� | �۵��е㣨���� | ˮ |
ˮ���� | 138 | 158���۵㣩 | �� |
������ | 102 | 139.4���е㣩 | ��ˮ�� |
����ˮ���� | 180 | 135���۵㣩 | �� |
�����������Ϣ�ش��������⣺
��1���Ʊ���˾ƥ��ʱ��Ҫʹ�ø����������ԭ����________________________________��
��2���ϳɰ�˾ƥ��ʱ��������ļ��ȷ�����______________________��

��3���ᴿ�ֲ�Ʒ�������£����Ȼ���װ����ͼ��
�ٷ�ʯ��������__________________________________��
������ˮ������������________________������b������c������
��ʹ���¶ȼƵ�Ŀ����_____________________________________________________��
��4����ʵ����ԭ��������2.0gˮ���ᡢ5.0mL��������![]() �������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ__________________���ðٷ�����ʾ��С�����һλ����
�������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ__________________���ðٷ�����ʾ��С�����һλ����
����Ŀ���������Ǻ���Ҫ�Ľ������ѳ�Ϊ������������Ҫ�IJ��ϡ��ش�����������
��1����̬��ԭ�ӵļ۵����Ų�ʽΪ__________________�������Ѿ���������ͼ��ʾ����������Ϊa=b= 295.08pm��c=468.55pm����=��=90%��y= 120%��������Ϊ______________�ѻ�(��ѻ���ʽ)��
��2�������ڵ�þ������л�ԭTiCl4�ɵõ���յĺ����ѡ���֪TiCl4��ͨ�����������ɫҺ�壬�۵�Ϊ-23�棬�е�Ϊ136�棬��֪TiCl4Ϊ____________�� �塣
��3��ͨ��X-����̽��KCl��CaO��TiN������NaCl����ṹ���ƣ���֪�������Ӿ���ľ���������������
���Ӿ��� | KCl | CaO |
������(kJ/mol) | 715 | 3401 |
����KCl������С��CaO��ԭ����_______________��
�ѿ���C��N��O��Ԫ���γɶ�Ԫ�����C��N��OԪ�صĵ縺���ɴ�С��˳����________��
(4)���ѿ���Ľṹ������ͼ��ʾ������Ļ�ѧʽΪ_________________��
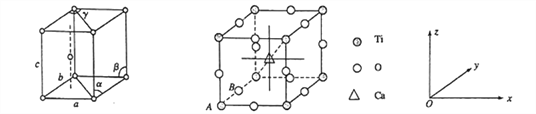
�����е�ԭ�ӿ���x��y��z��ɵ����������������ھ����е�λ�ã���Ϊԭ�����ꡣ��֪ԭ������ΪA(0��0��0)��B(0��1/2��0)����Ca ���ӵ�ԭ������Ϊ______________��
��5��Fe���ġ��á�������ͬ�������壬�侧���ṹ����ͼ��ʾ��
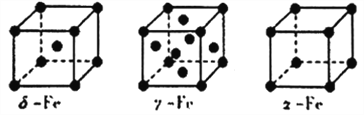
�٦ġ������־��徧������ԭ�ӵ���λ��֮��Ϊ_______________________��
����Feԭ�Ӱ뾶Ϊrpm��NA��ʾ�����ӵ�������ֵ������-Fe���ʵ��ܶ�Ϊ________g/cm3(�г���ʽ����)��

